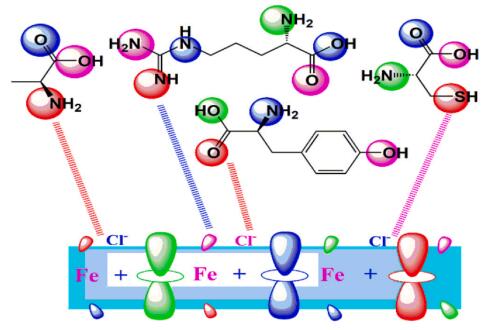
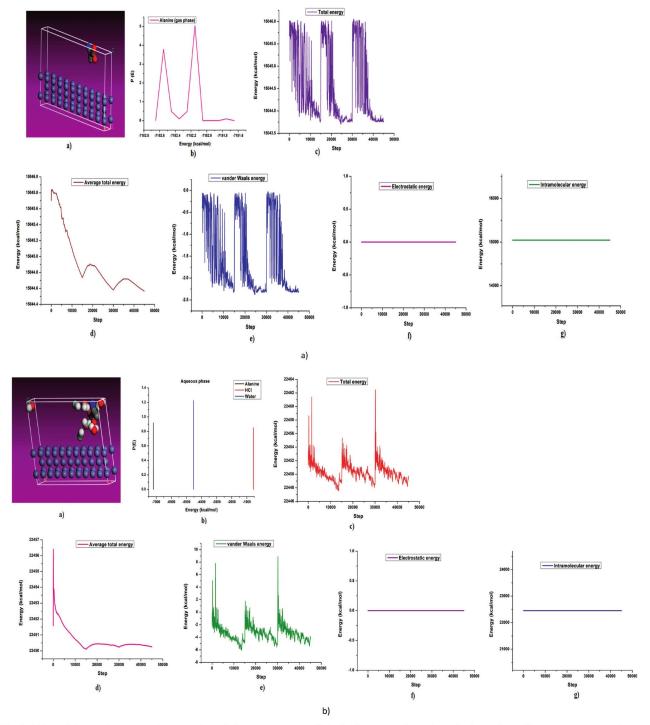
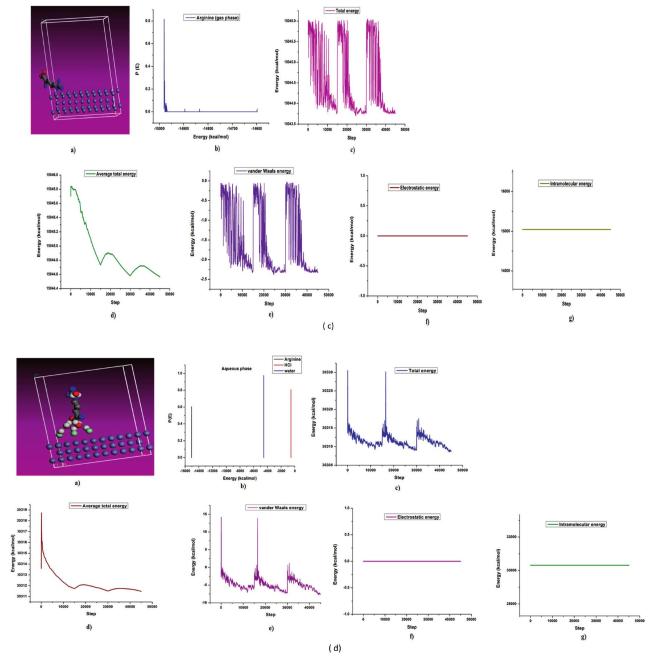
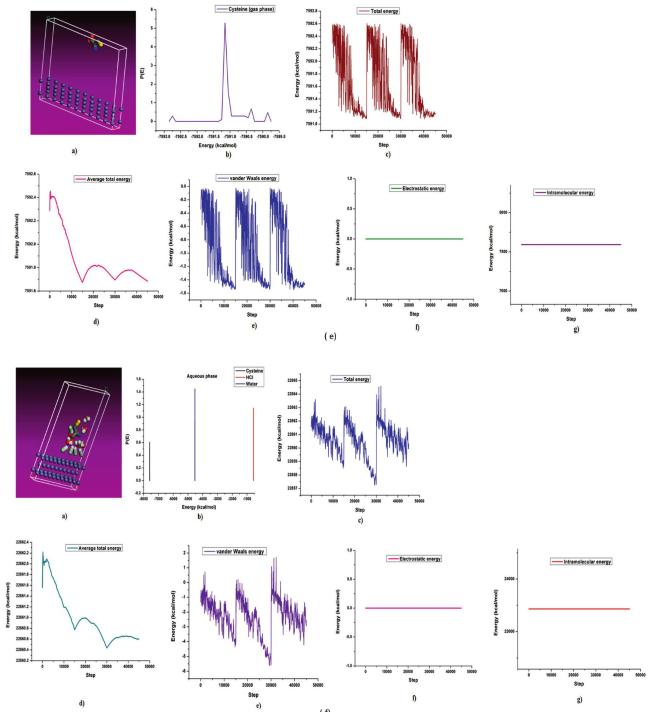
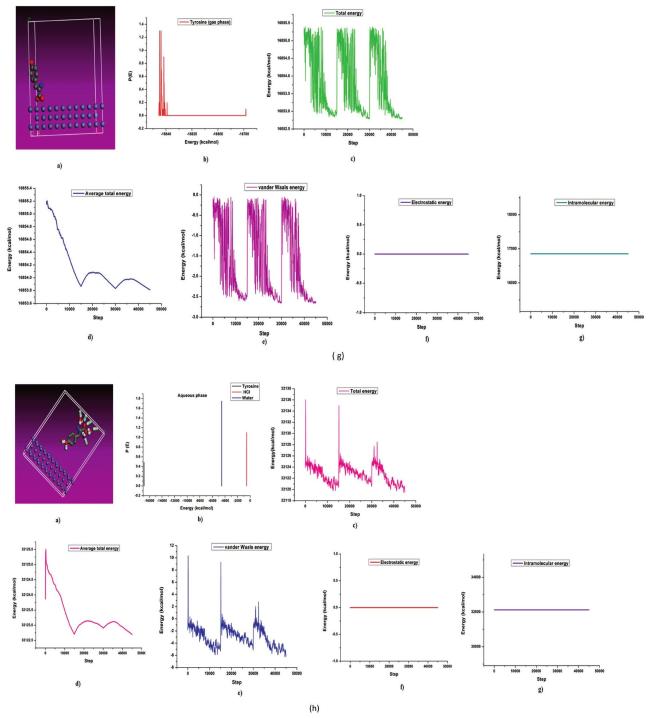
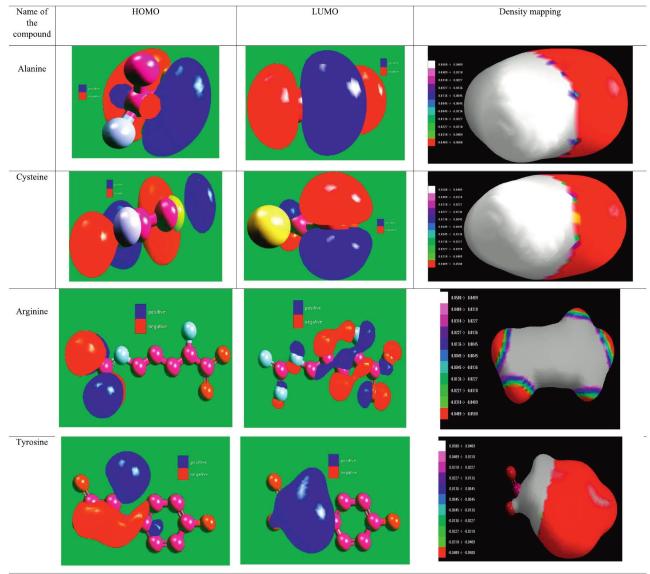
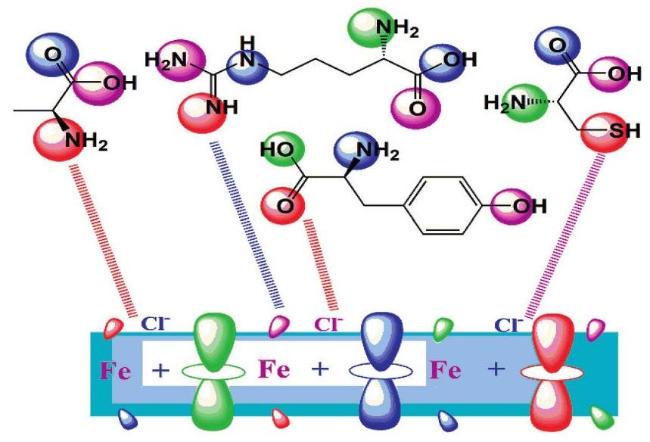
The commonly used method for determining a compound's reactivity is molecular orbital occupation, or DFT, which looks at both high- and low-occupied orbitals. According to frontier molecular orbital theory, the lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) of a corrosion inhibitor can reflect its morphology and adsorption site, which is important information for determining the inhibitor's inhibitory activity [
37,
38]. Numerous variables were calculated, such as the ionisation potential (I), chemical potential (
$\mu $ ), electron affinity (A), chemical hardness (
$\eta $ ), chemical softness or electron polarizability (
$\sigma $ ), electronegativity (
$\chi $ ), electrophilicity index (
$\omega $ ), nucleophilicity (
$\epsilon =1/\omega $ ) energy of lowest unoccupied molecular orbital (ELUMO), energy of highest occupied molecular orbital (EHOMO), and electron affinity (A). Compounds that effectively inhibit and stop corrosion processes are measured by the energy (
$\mathrm{\Delta }\mathrm{E}$ ) difference between their LUMO and HOMO values. Therefore, the more effectively inhibition occurs, the less energy is
required to remove an electron from the most recently occupied orbit. Energy also acts as a barometer for molecular stability; a lower
$\mathrm{\Delta }\mathrm{E}$ value denotes the emergence of a more stable complex on the metal surface [
39,
40]. The energy gap (
$\mathrm{\Delta }\mathrm{E}$ ), which is significant for molecules' electron transport properties. Smaller
$\mathrm{\Delta }\mathrm{E}$ values are linked to stronger corrosion resistance and increased interaction between the inhibitor and mild steel [
37]. In
Fig. 3 and
Table 3, the corresponding quantum chemical parameters are shown. Nonetheless, a substantial body of literature has established a strong relationship between the LUMO-HOMO energy gap (
△Egap ) and the chemical reactivity of the molecules, with a soft molecule having a small value and a hard molecule having a large value [
41,
42]. When the orbital is full of electrons, more inhibition is generated and the material donates readily to the metal surface. Conversely, when the low unoccupied orbital value is small, there should be a good donation from the metal surface to the substance. Inhibitors react more strongly when
$\mathrm{\Delta }\mathrm{E}$ is lower [
43-
47]. The Cysteine and Tyrosine molecule in this case has an
$\mathrm{\Delta }\mathrm{E}$ of 7.49 and 7.74 eV, whereas the Alanine and Arginine molecules have
$\mathrm{\Delta }\mathrm{E}$ values of 8.9 and 8.04 eV, respectively. Based on these numbers, it appears that the Cysteine and Tyrosine molecules is highly reactive. It is noteworthy that, the Cysteine and Tyrosine molecule inhibitor consistently displays the lowest
$\mathrm{\Delta }\mathrm{E}$ value for the various hybrid functionals, indicating a high reactivity and efficiency in comparison to the two remaining compounds. The general reactivity and stability of the molecules are assessed using global hardness and electronegativity. The reactivity and ease of electron release increase with decreasing global hardness value. Furthermore, a higher value of overall softness indicates better performance in corrosion inhibition. High chemical reactivity and high inhibition efficiency are usually seen in inhibitors with low global hardness and high softness values. The calculated data indicate that the low hardness and high global softness of the cysteine and tyrosine molecule cause a high inhibition on the Fe (110) surface. Electrons are donated back to the metal surface through the interaction of the molecule. Strong molecules with a soft chemical makeup are effective corrosion inhibitors. This suggests that there is little difficulty in the soft molecule and the metal surface sharing electrons.