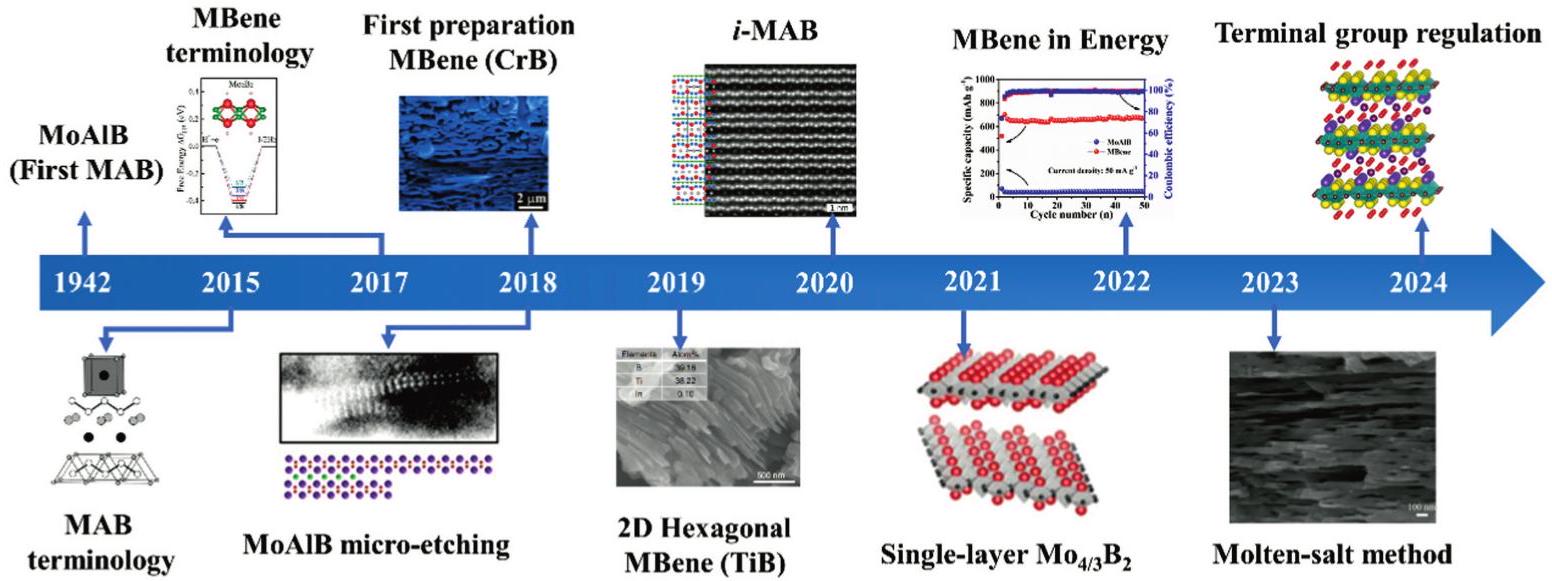
 Fig. 2. The representative historical timeline of MBenes. Copyright 2015 [
Fig. 2. The representative historical timeline of MBenes. Copyright 2015 [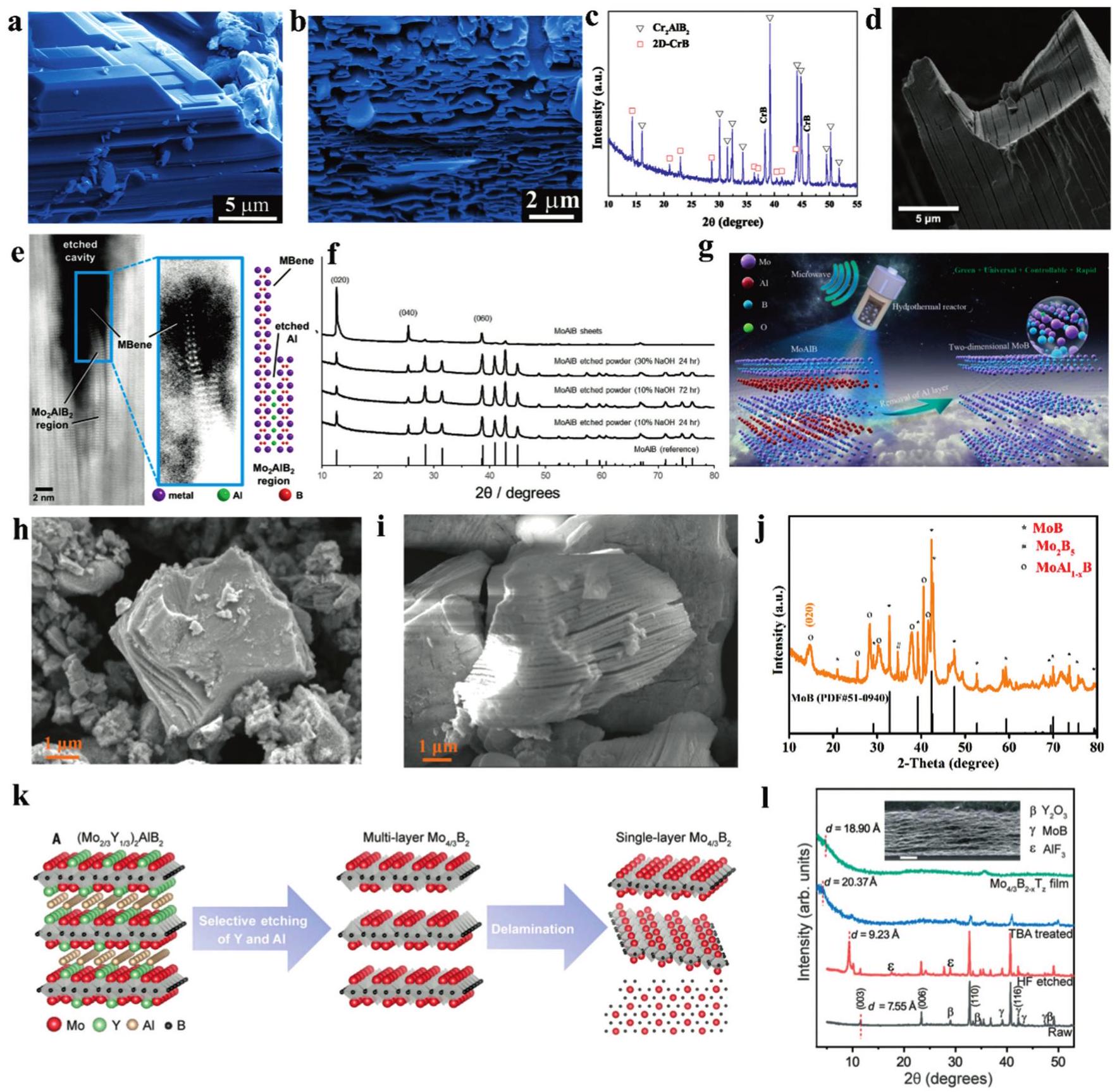 Fig. 3. SEM images of (a) Cr2AlB2, (b) CrB MBene etched by diluted HCl, (c) XRD patterns of CrB MBene and Cr2AlB2. (d) SEM image of etched MoAlB soaked into 10%NaOH for 24 h, (e) ADF-STEM image of delaminated MoB sheets in an etched area, (f) XRD patterns bulk MoAlB and etched MoAlB soaked into NaOH solutions. (g) The illustration of the MoB MBene prepared by the microwave-assisted hydrothermal alkaline solution method. Reproduced with permission from Ref. [
Fig. 3. SEM images of (a) Cr2AlB2, (b) CrB MBene etched by diluted HCl, (c) XRD patterns of CrB MBene and Cr2AlB2. (d) SEM image of etched MoAlB soaked into 10%NaOH for 24 h, (e) ADF-STEM image of delaminated MoB sheets in an etched area, (f) XRD patterns bulk MoAlB and etched MoAlB soaked into NaOH solutions. (g) The illustration of the MoB MBene prepared by the microwave-assisted hydrothermal alkaline solution method. Reproduced with permission from Ref. [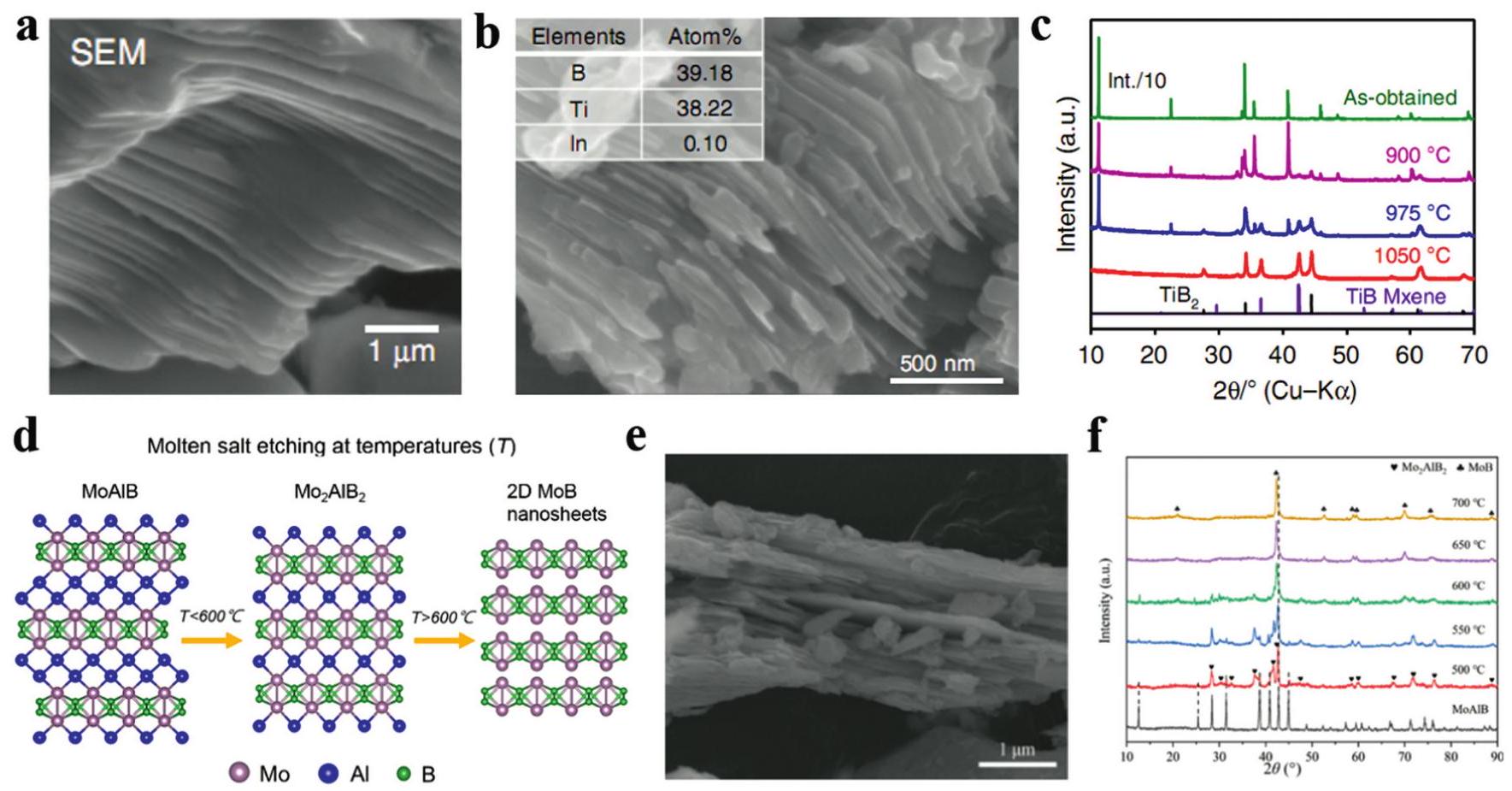 Fig. 5. SEM images of (a) Ti2InB2, (b) layered TiB and its atomic ratio. (c) XRD patterns of layered TiB etched at different temperature. (d) The shematic illustration of MoB MBene etched at different temperature, (e) SEM image of as-prepared MoB MBene, (f) XRD patterns of MoAlB etched by ZnCl2 at different temperature. Reproduced with permission from Ref [
Fig. 5. SEM images of (a) Ti2InB2, (b) layered TiB and its atomic ratio. (c) XRD patterns of layered TiB etched at different temperature. (d) The shematic illustration of MoB MBene etched at different temperature, (e) SEM image of as-prepared MoB MBene, (f) XRD patterns of MoAlB etched by ZnCl2 at different temperature. Reproduced with permission from Ref [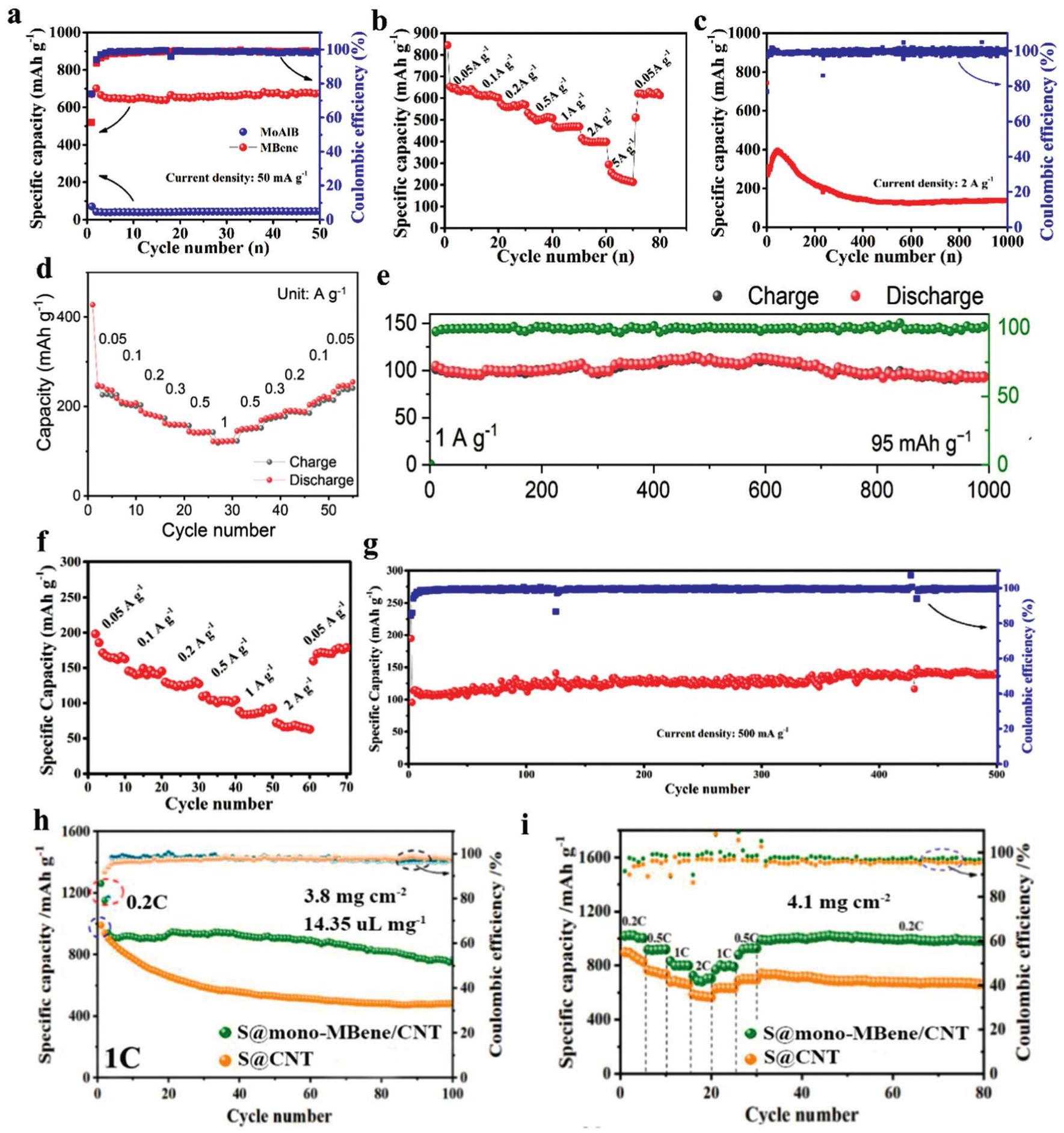 Fig. 6. (a) The cycle performance of MoAlB and MoB MBene at 50mAg-1 in LIBs, (b) rate capability and (c) long cycle performance at 2Ag-1 of MoB MBene. (d) Rate capability and (e) long cycle performance of the HfB MBene anode in LIBs. (e) Rate capability and (f) long cycle performance of the MoB MBene anode in SIBs. (h) Comparison of the cyclic capacity at 1 C and (i) the cyclic capacities at variant rates ranging from 0.2 C to 2 C of S@mono-MBene/CNT and S@CNT cathodes. Reproduced with permission from Ref [
Fig. 6. (a) The cycle performance of MoAlB and MoB MBene at 50mAg-1 in LIBs, (b) rate capability and (c) long cycle performance at 2Ag-1 of MoB MBene. (d) Rate capability and (e) long cycle performance of the HfB MBene anode in LIBs. (e) Rate capability and (f) long cycle performance of the MoB MBene anode in SIBs. (h) Comparison of the cyclic capacity at 1 C and (i) the cyclic capacities at variant rates ranging from 0.2 C to 2 C of S@mono-MBene/CNT and S@CNT cathodes. Reproduced with permission from Ref [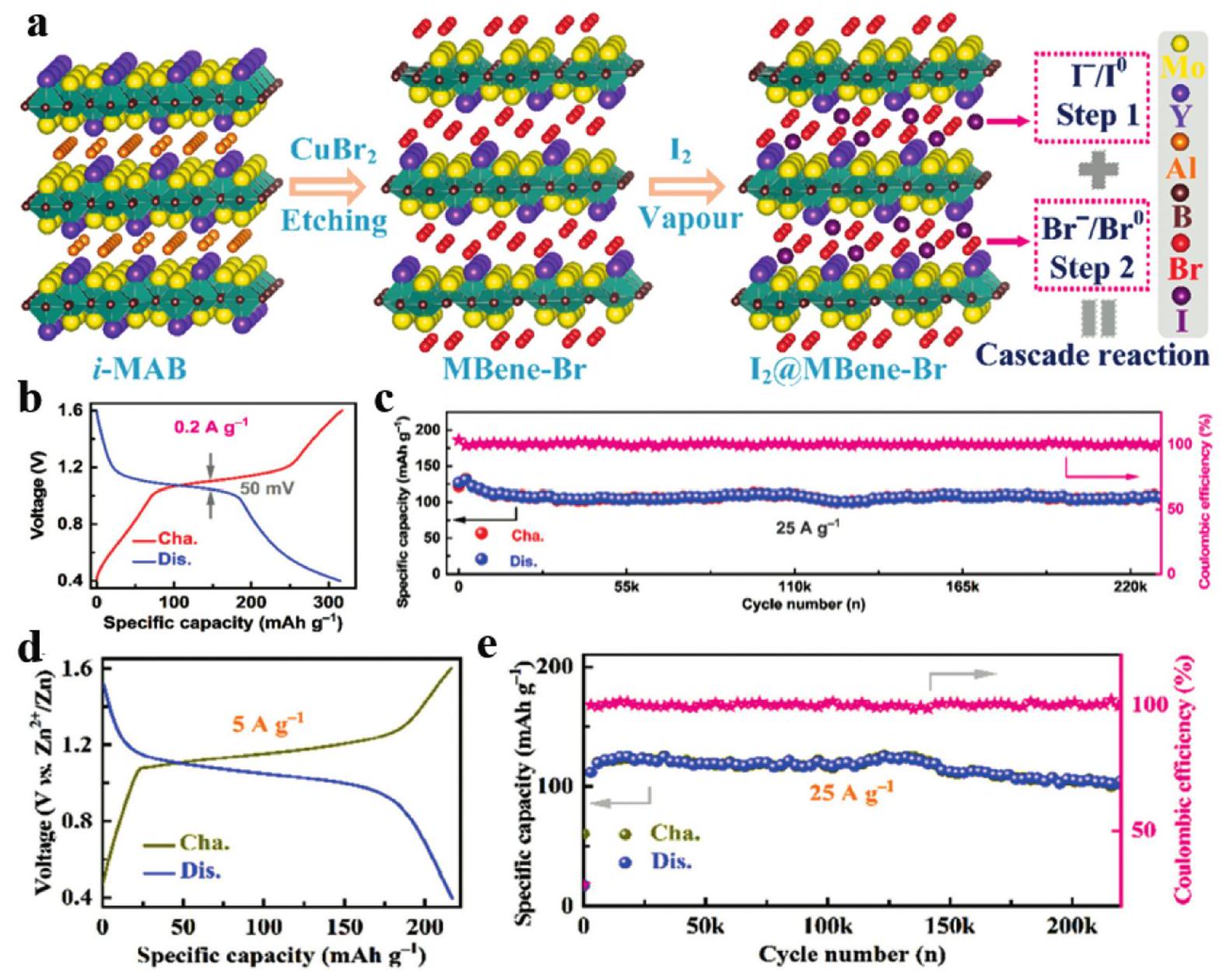 Fig. 7. (a) Schematic illustration of the preparation of the I2@MBene-Br electrode and the cascade reaction. (b) The charge and discharge profile at 0.2Ag-1 and (c) the long-cycle performance at 25Ag-1 of Zn-I2 batteries. (d) The charge and discharge profile at 5Ag-1 and (e) the cycling performance of the I2@MBene-Br electrode. Reproduced with permission from Ref [
Fig. 7. (a) Schematic illustration of the preparation of the I2@MBene-Br electrode and the cascade reaction. (b) The charge and discharge profile at 0.2Ag-1 and (c) the long-cycle performance at 25Ag-1 of Zn-I2 batteries. (d) The charge and discharge profile at 5Ag-1 and (e) the cycling performance of the I2@MBene-Br electrode. Reproduced with permission from Ref [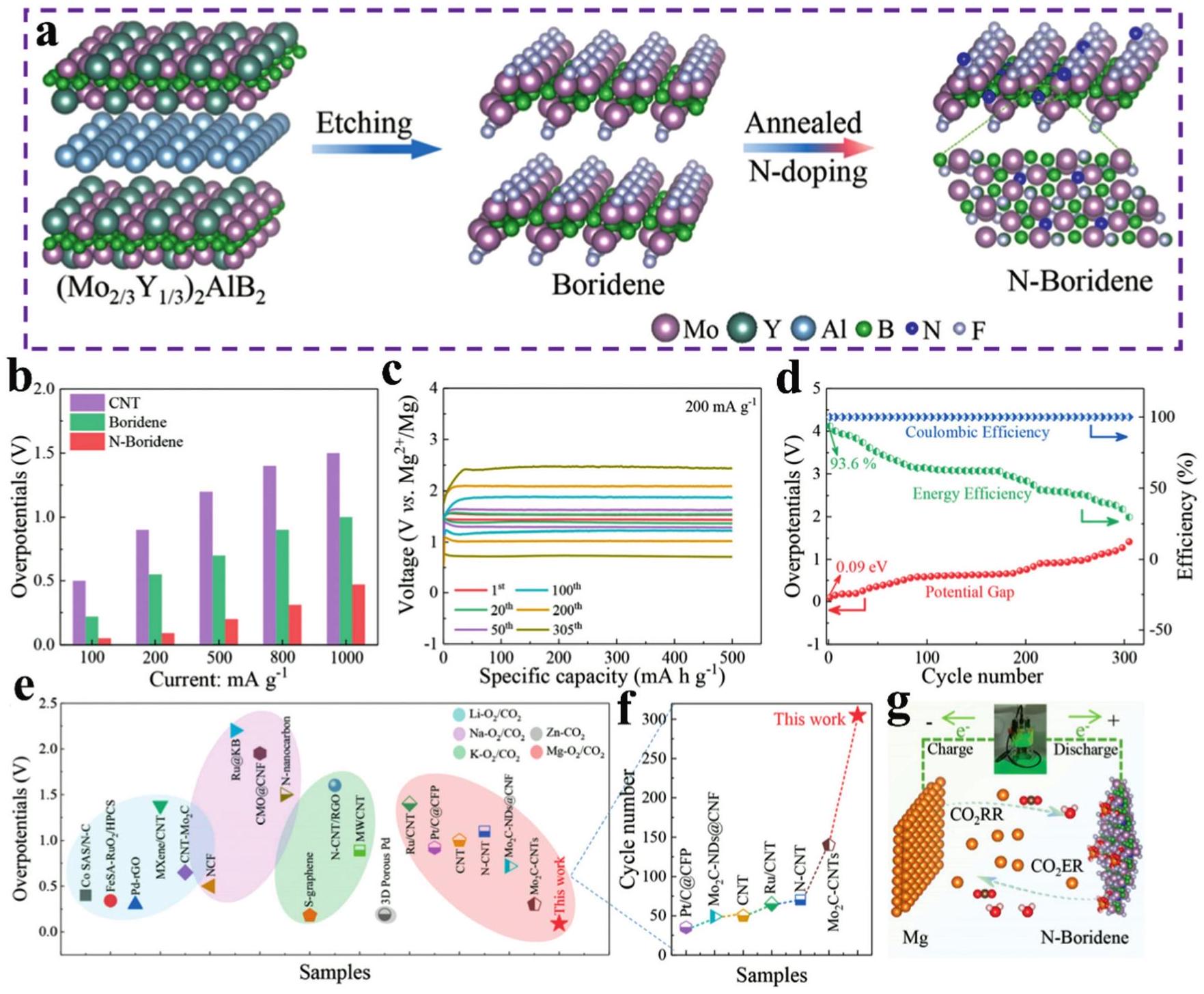 Fig. 8. (a) Schematic illustration for the synthesis of N -boridene. (b) The initial-circle overpotentials for the three catalysts at different current densities. (c) The electrochemical performance of N -boridene batteries at a current density of 200mAhg-1 with a discharge and charge depth of 500mAhg-1. (d) Overpotentials, Coulombic efficiency and energy efficiency during the 305 cycles. (e, f) Comparison of the overpotentials and cycle numbers between N-boridene batteries and reported work. (g) Photo figure of LED lighted by the N-boridene-based Mg-CO2 batteries. Reproduced with permission from Ref. [
Fig. 8. (a) Schematic illustration for the synthesis of N -boridene. (b) The initial-circle overpotentials for the three catalysts at different current densities. (c) The electrochemical performance of N -boridene batteries at a current density of 200mAhg-1 with a discharge and charge depth of 500mAhg-1. (d) Overpotentials, Coulombic efficiency and energy efficiency during the 305 cycles. (e, f) Comparison of the overpotentials and cycle numbers between N-boridene batteries and reported work. (g) Photo figure of LED lighted by the N-boridene-based Mg-CO2 batteries. Reproduced with permission from Ref. [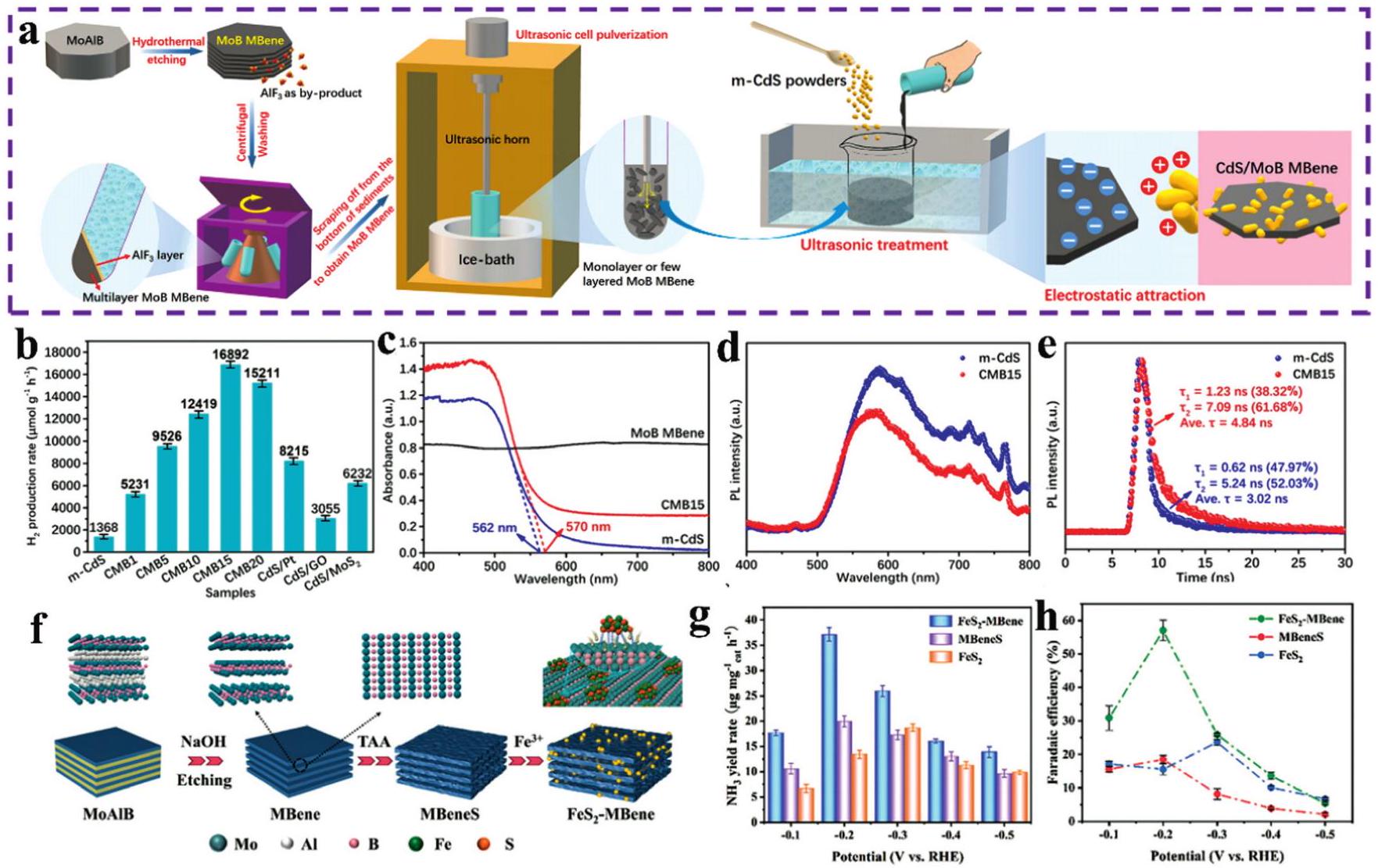 Fig. 9. (a) Schematic procedure of the preparation of MoB MBene and the composite of CdS/MoB MBene. (b) The hydrogen production rate of CMBx, m-CdS, CdS/Pt, CdS/GO and CdS/MoS 2. (c) Ultraviolet-visible diffuse reflectance spectra of m-CdS. (d) Steady-state and (e) time-resolved PL spectra of m-CdS and CMB15 in photocatalysis. (f) Synthetic illustration of FeS2-MBene. (g) The yield rates of NH3. (h) Faradaic efficiency of FeS2, MBeneS and FeS2-MBene in electrochemical catalysis. Reproduced with permission from Ref [
Fig. 9. (a) Schematic procedure of the preparation of MoB MBene and the composite of CdS/MoB MBene. (b) The hydrogen production rate of CMBx, m-CdS, CdS/Pt, CdS/GO and CdS/MoS 2. (c) Ultraviolet-visible diffuse reflectance spectra of m-CdS. (d) Steady-state and (e) time-resolved PL spectra of m-CdS and CMB15 in photocatalysis. (f) Synthetic illustration of FeS2-MBene. (g) The yield rates of NH3. (h) Faradaic efficiency of FeS2, MBeneS and FeS2-MBene in electrochemical catalysis. Reproduced with permission from Ref [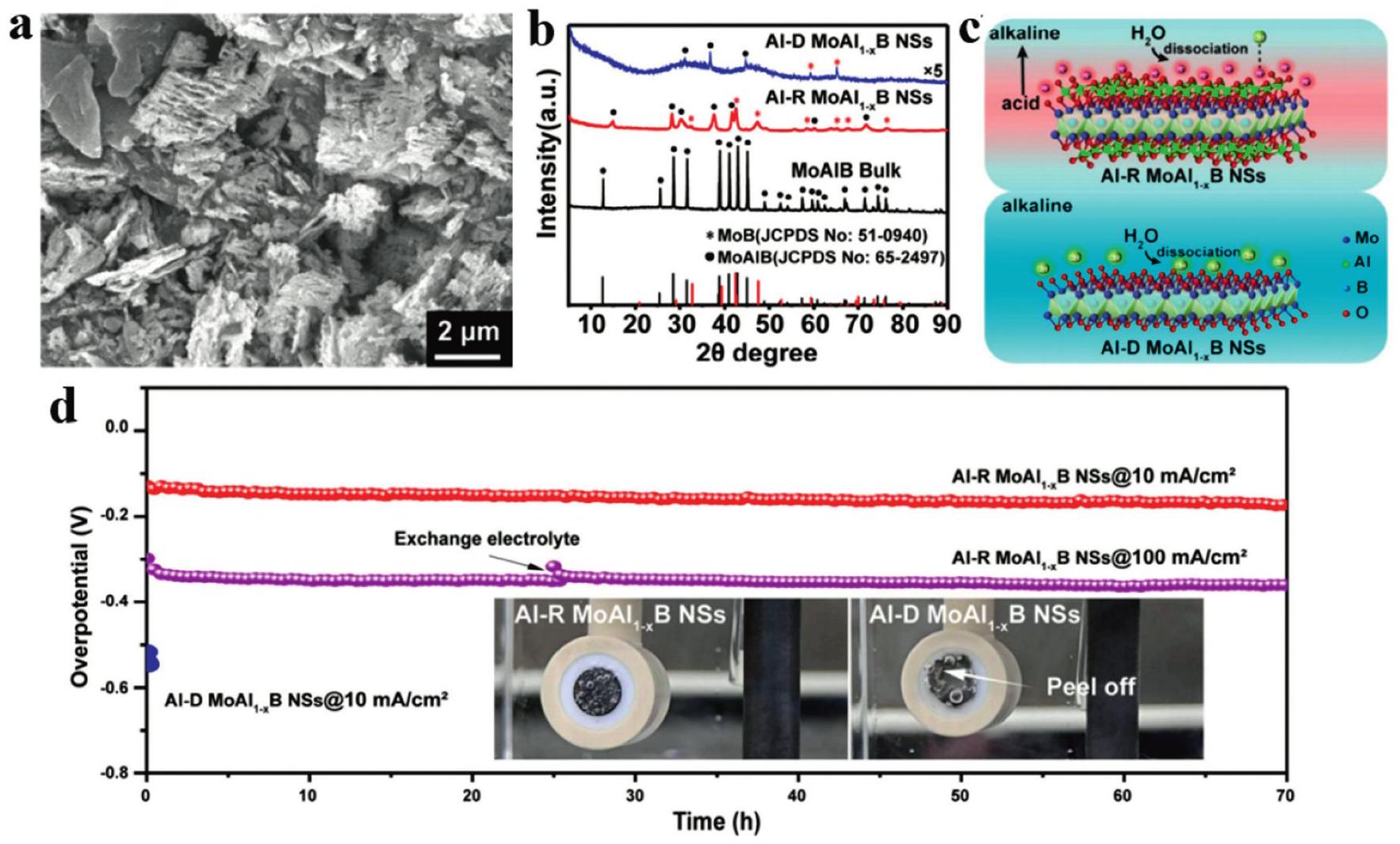 Fig. 10. (a) SEM image of the Al-R MoAl1-xB, (b) XRD patterns of the etched samples of Al-R and Al-DMoAl 1-xB, (c) schematic illustration of the microenvironment between Al-R and Al-D MoAl 1-x B samples, (d) chronopotentiometry test of Al-RMoAl1-x B recorded at the current densities of 10 and 100 mA cm -2, the inset image illustrates the hydrogen gas bubbles on the surface of Al-R and Al-DMoAl1-xB electrodes. Reproduced with permission from Ref [
Fig. 10. (a) SEM image of the Al-R MoAl1-xB, (b) XRD patterns of the etched samples of Al-R and Al-DMoAl 1-xB, (c) schematic illustration of the microenvironment between Al-R and Al-D MoAl 1-x B samples, (d) chronopotentiometry test of Al-RMoAl1-x B recorded at the current densities of 10 and 100 mA cm -2, the inset image illustrates the hydrogen gas bubbles on the surface of Al-R and Al-DMoAl1-xB electrodes. Reproduced with permission from Ref [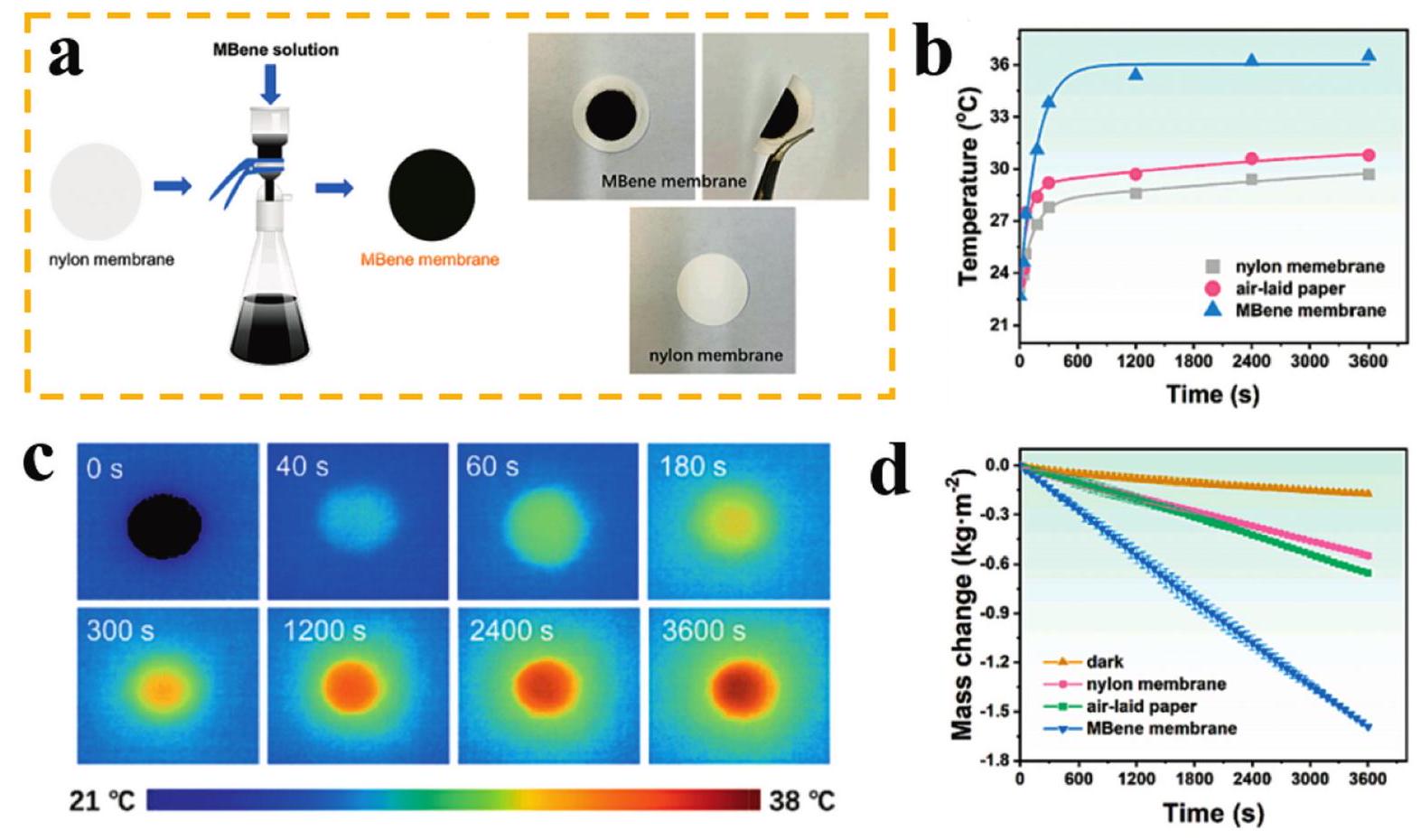 Fig. 11. (a) Schematic illustration and photographs of MBene membrane and nylon membrane. (b) The temporal variation of the surface temperature, (c) Infrared thermal figures of MBene membrane, (d) The temporal variation of water mass change of the three evaporation models under 1 sun irradiation. Reproduced with permission from Ref. [
Fig. 11. (a) Schematic illustration and photographs of MBene membrane and nylon membrane. (b) The temporal variation of the surface temperature, (c) Infrared thermal figures of MBene membrane, (d) The temporal variation of water mass change of the three evaporation models under 1 sun irradiation. Reproduced with permission from Ref. [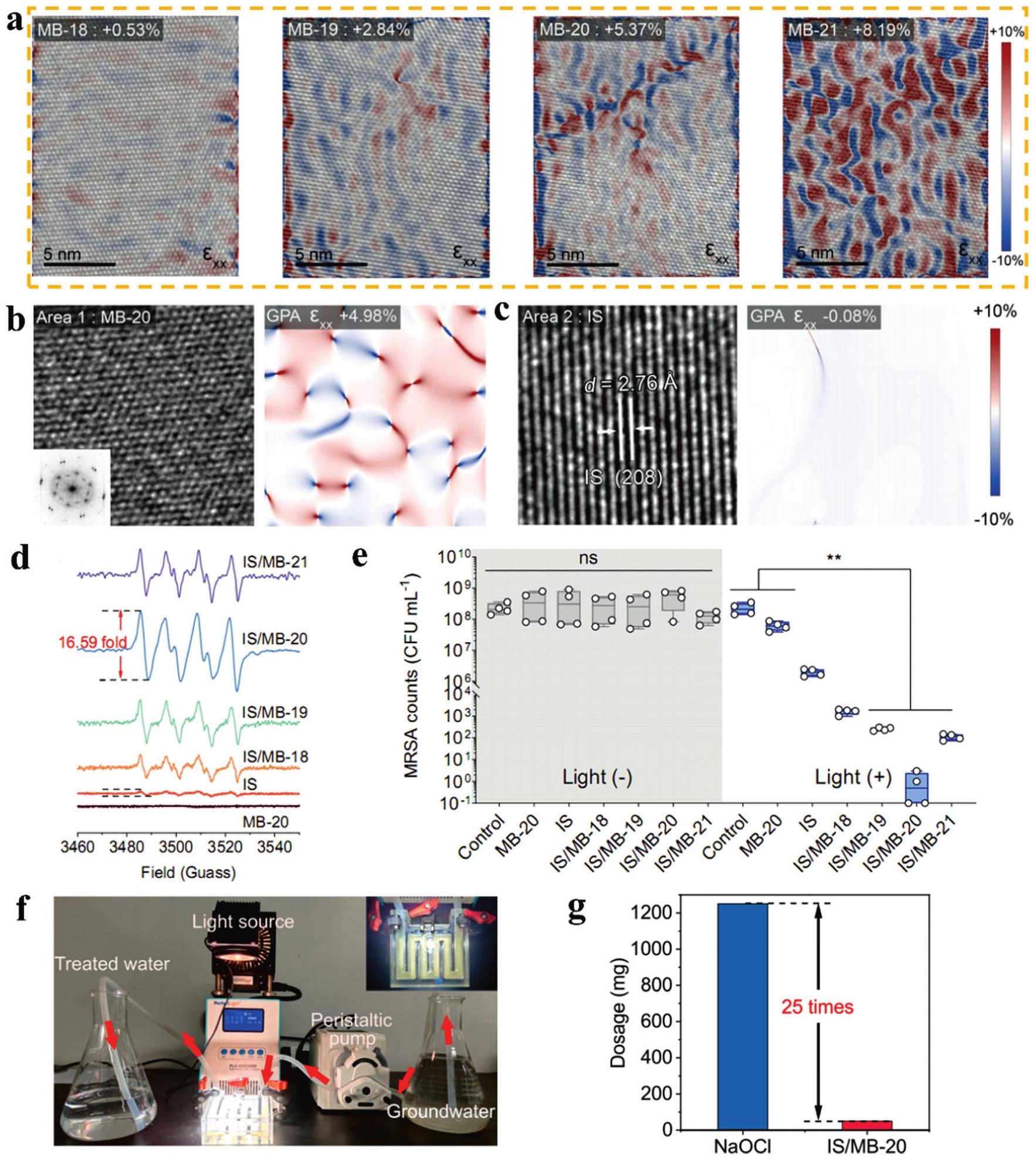 Fig. 12. (a) AC-STEM and GPA images of Mo4/3 B2-xTz MBene (MB) etched by HF at different time (18-21 h) (b) and (c) HRTEM images and corresponding strain mapping images based on GPA of MB-20 and IS. (d) In-situ EPR spectra of O2 -generated by different samples in low dissolved oxygen condition under visible light irradiation. (e) Quantitative analysis of antibacterial effect (MRSA) at visible light irradiation. (f) The digital image of the continuous flow photocatalytic disinfection device. (g) The dosage of NaClO and IS/MB-20 for producing 37.2 L of clean water. Reproduced with permission from Ref. [
Fig. 12. (a) AC-STEM and GPA images of Mo4/3 B2-xTz MBene (MB) etched by HF at different time (18-21 h) (b) and (c) HRTEM images and corresponding strain mapping images based on GPA of MB-20 and IS. (d) In-situ EPR spectra of O2 -generated by different samples in low dissolved oxygen condition under visible light irradiation. (e) Quantitative analysis of antibacterial effect (MRSA) at visible light irradiation. (f) The digital image of the continuous flow photocatalytic disinfection device. (g) The dosage of NaClO and IS/MB-20 for producing 37.2 L of clean water. Reproduced with permission from Ref. [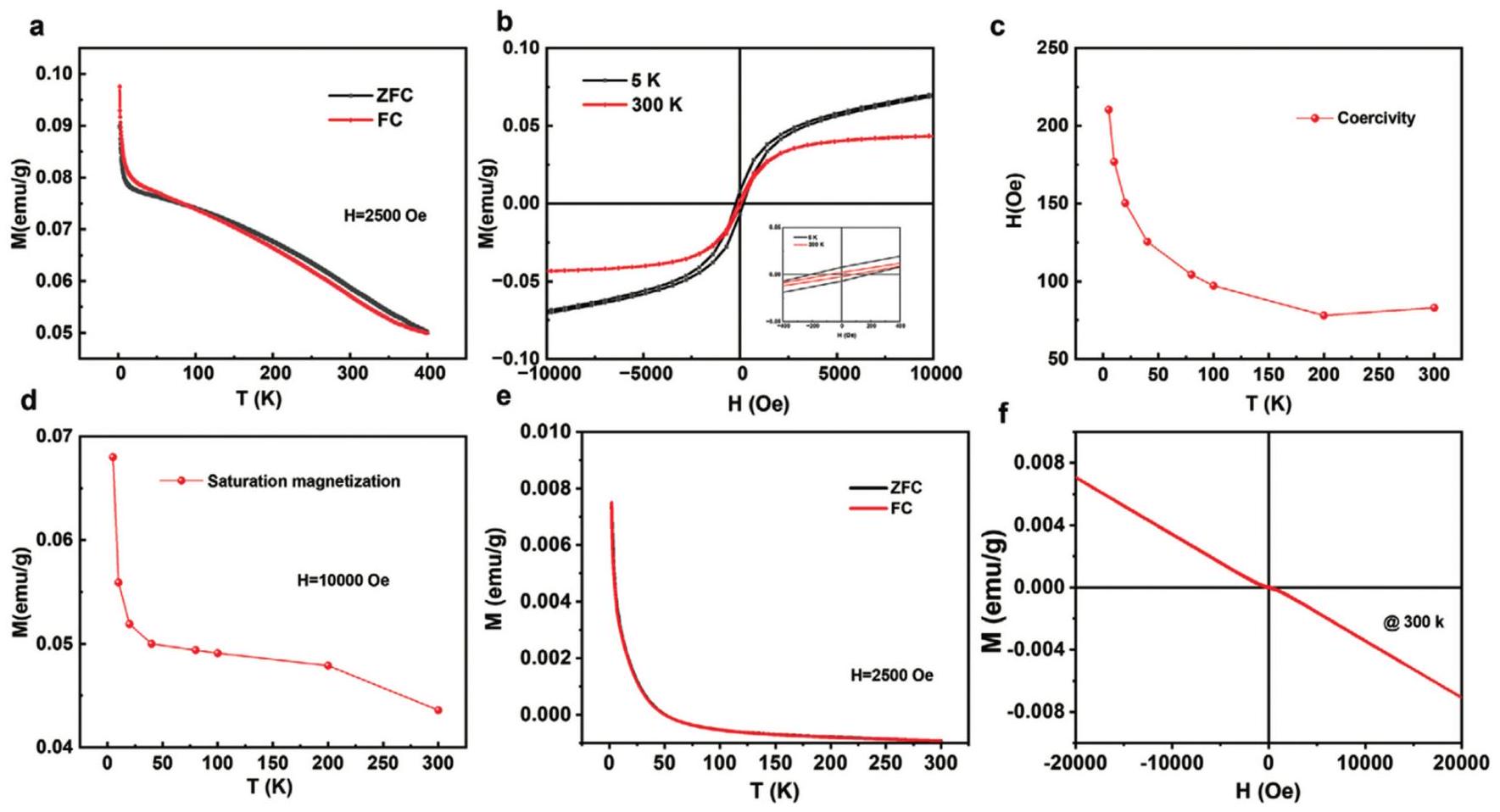 Fig. 13. (a,e) ZFC and FC magnetization plots of Mo4/3 B2 nanosheets and pristine MoB characterized under external field of 2500 Oe at different temperature, respectively. (b,f) Magnetization loops of Mo4/3 B2 at 5 and 300 K and MoB MBene at 300 K, respectively. (The enlarged view of the hysteresis loop in the inset). (c) Coercivity of Mo4/3 B2 at different temperatures. (d) Saturation magnetization plot of Mo4/3 B2. Reproduced with permission from Ref. [
Fig. 13. (a,e) ZFC and FC magnetization plots of Mo4/3 B2 nanosheets and pristine MoB characterized under external field of 2500 Oe at different temperature, respectively. (b,f) Magnetization loops of Mo4/3 B2 at 5 and 300 K and MoB MBene at 300 K, respectively. (The enlarged view of the hysteresis loop in the inset). (c) Coercivity of Mo4/3 B2 at different temperatures. (d) Saturation magnetization plot of Mo4/3 B2. Reproduced with permission from Ref. [