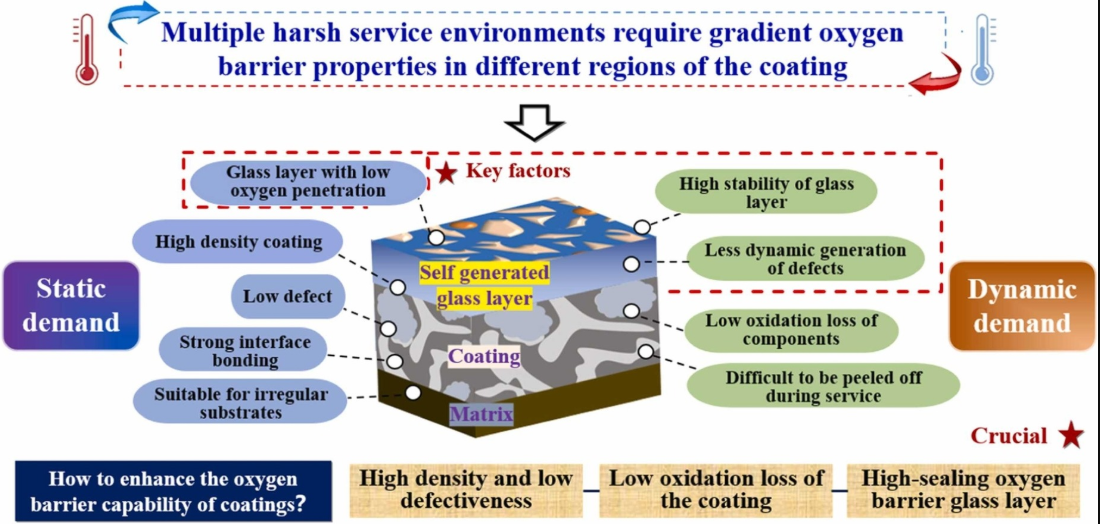
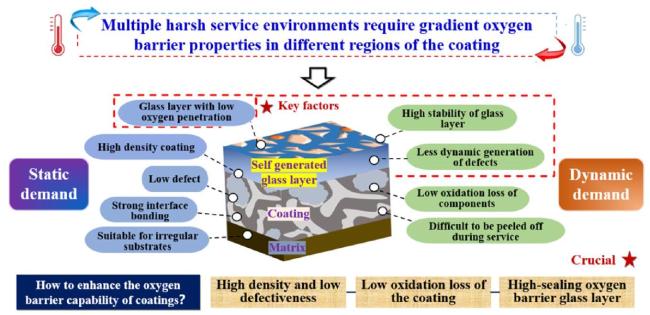
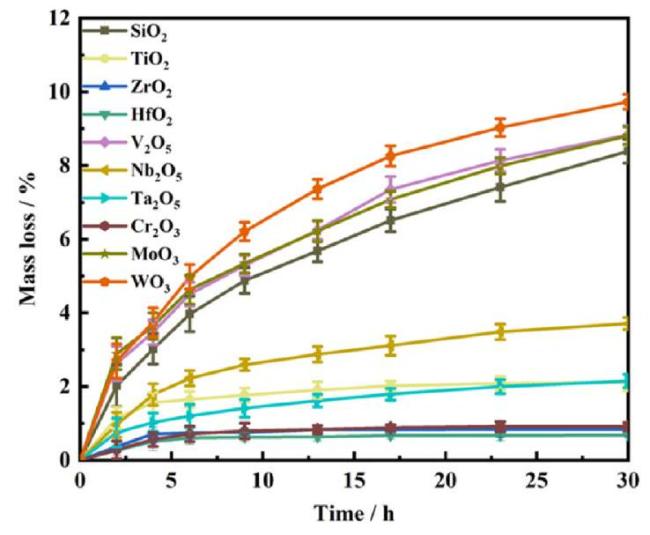
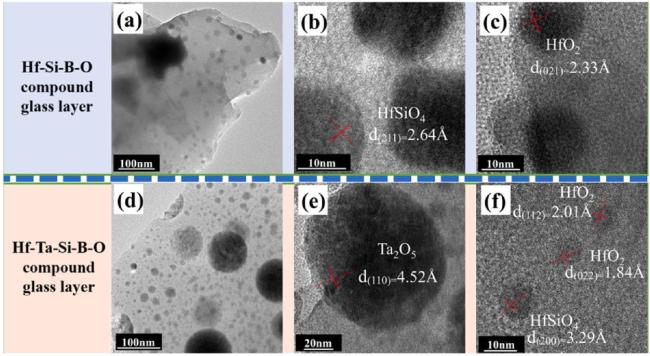
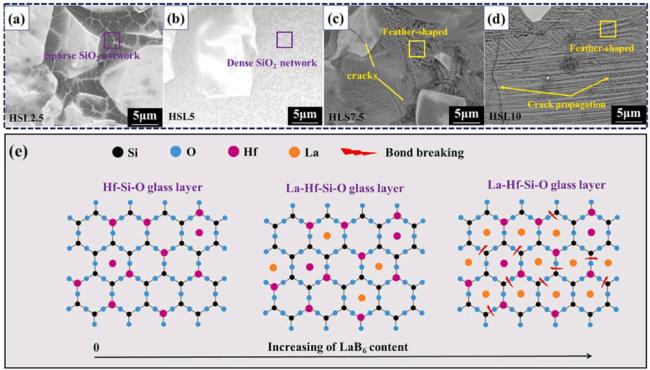
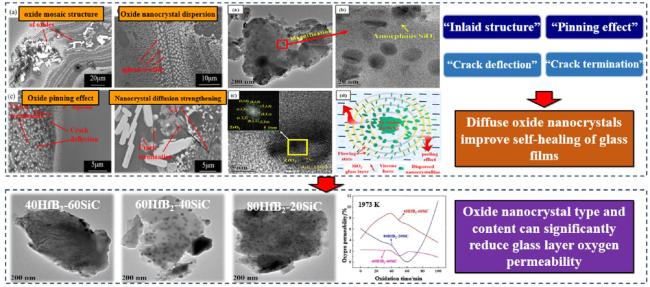
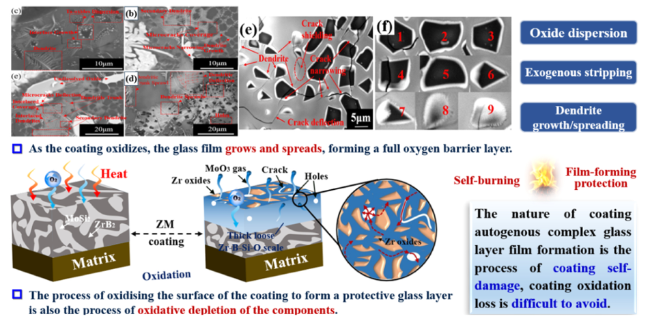
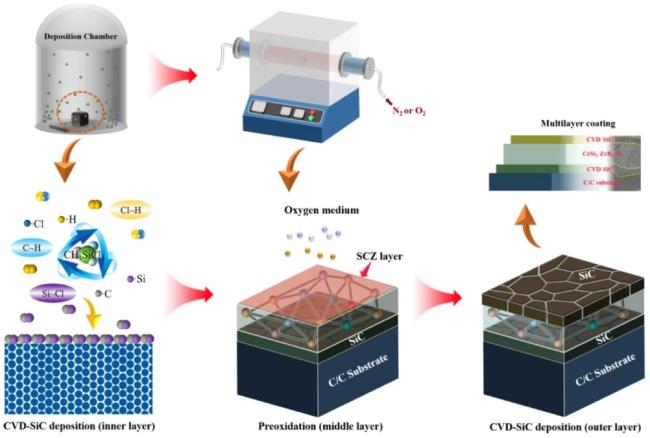
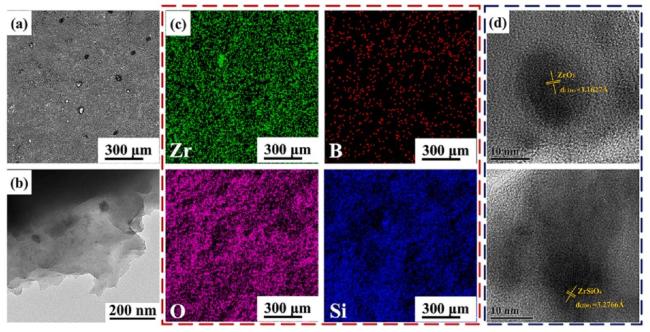
1. Introduction
Fig. 1. Gradient high oxygen barrier concept of UHTC coatings. Reproduced with permission from Ref. [13], © Elsevier 2024. |
2. Coating structures
2.1. Single-layer coatings
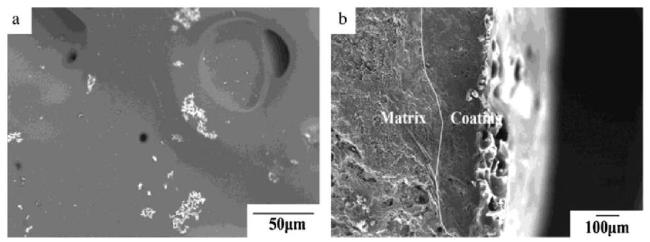
Fig. 2. SEM images of SiC coatings with a Si/C weight ratio of 6:0.5 after 100 h of oxidation in air at 1500∘C : (a) surface; (b) cross-section. Reproduced with permission from Ref. [22], © Elsevier 2015. |
2.2. Double-layer coatings
2.3. Gradient coatings
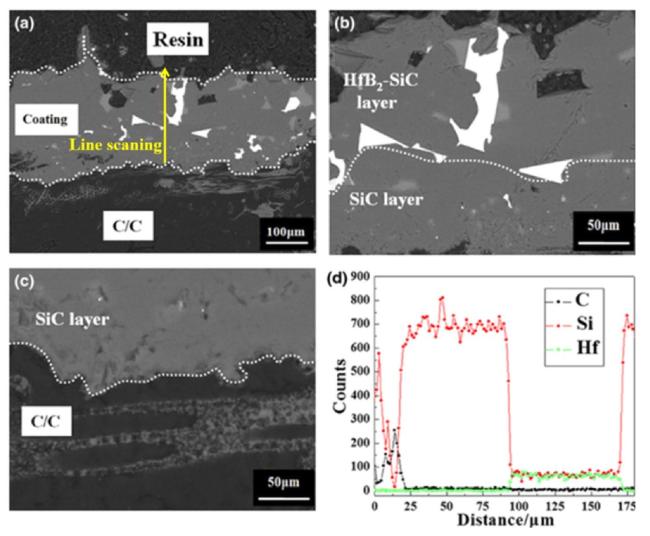
Fig. 3. (a) Backscattering cross-section micrographs of coated C/C composites; High-magnification backscattered electron micrograph of the (b) coating and (c) interface between the C/C composite and the internal SiC layer; (d) Energy dispersive spectroscopy elemental line analysis of the coating in (a), with the yellow arrow representing the scanning direction. Reproduced with permission from Ref. [33], © Wiley-Blackwell 2014. |
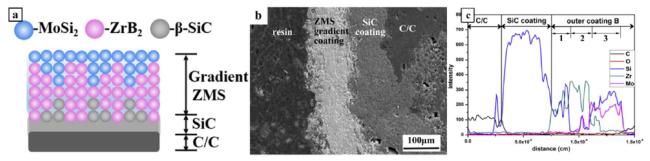
Fig. 4. (a) Schematic diagram of the sprayed gradient SZM coating on SiC-coated C/C composites, (b) cross-sectional morphology, and (c) elemental line scan. Reproduced with permission from Ref. [36], © Elsevier 2017. |
2.4. Nano toughened coating
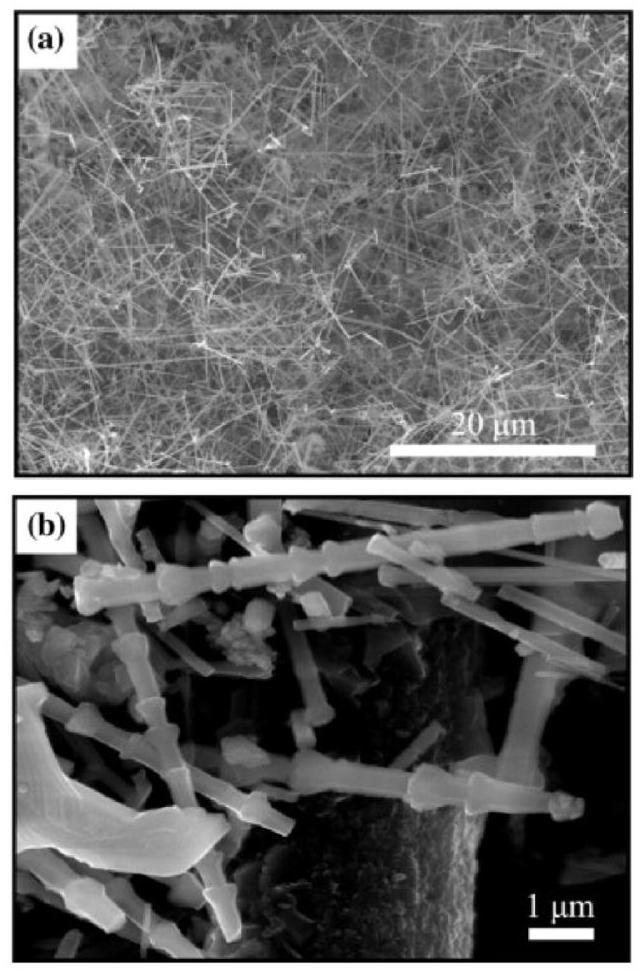
Fig. 5. SEM images of the bamboo-like SiC nanowires with a porous network on the surface of C/C samples: (a) low magnification; (b) high magnification.Reproduced with permission from Ref. [41], © Elsevier 2013. |
3. Preparation methods of the coatings
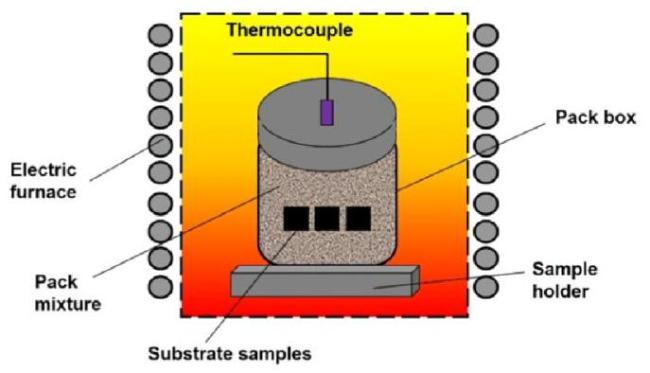
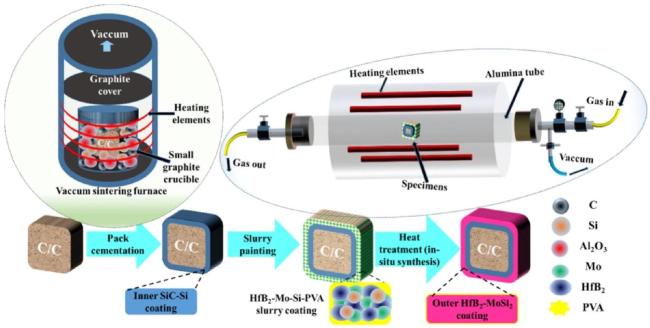
3.1. Pack cementation method
Fig. 6. (a) TEM image of a single SiC nanowire; (b) HRTEM image of the core-shell interface; (c) Elemental mapping of the nanowire; HADDF image of (d) nanowires surrounding ZrB2 and (e) the interface between ZrB2 and SiC. Reproduced with permission from Ref. [42], © Tsinghua University Press 2024. |
Fig. 7. SEM microstructure of SiCw-HfB2-SiC-Si/SiC coating: (a) cross-section; (b-c) cross-sectional SiCw bridging; (d) cross-sectional SiCw pull-out and debonding.Reproduced with permission from Ref. [46], © Elsevier 2018. |
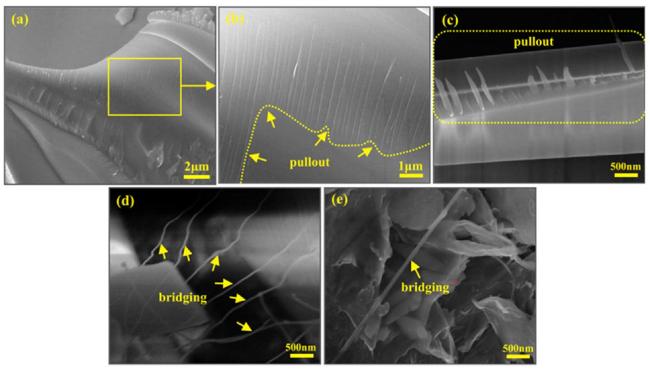
Fig. 8. Fracture surface SEM images of the SiCnws-LMS/SiC coating: (a-c) the representative nanowire pull-out feature; (d, e) the representative nanowire bridging feature. Reproduced with permission from Ref. [48], © Elsevier 2018. |
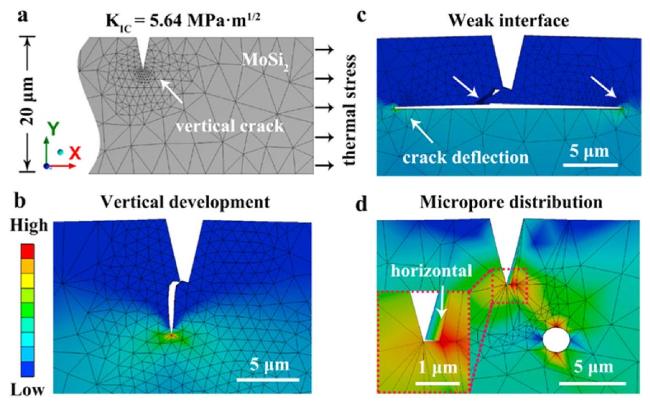
Fig. 9. Vertical crack development behavior under different conditions calculated by the smart crack growth modules of ANSYS: (a) the vertical crack development model; (b) the vertical development behavior in MoSi2 under the action of thermal stress; (c) the crack deflection behavior with weak interface; (d) the crack steering induced by micropore distribution. Reproduced with permission from Ref. [49], © Elsevier 2021. [49], © Elsevier 2021. |
Table 1 High temperature oxidation resistance of single layer coating, double layer coating, gradient coating and nanomaterial coating. |
| Coating materials | Fabrication methods | Temperature ( ∘C ) | Time (h) | Mass loss (wt%) | Refs. |
|---|---|---|---|---|---|
| ZrB2-ZrC-SiC | LC | 1500 | 0.8 | 0.51 g/cm2 | [21] |
| SiC | PEM + CVI | 1500 | 100 | 7.75 g/cm2 | [22] |
| B4C-HfB2-SiC | ISR | 1200 | 104 | 5.45 % | [23] |
| ZrB2-SiC | LPS | 1500 | 200 | 0.14 % | [25] |
| ZrB2 | SCC | 1500 | 342 | 1.03 % | [28] |
| ZrB2-CrSi2-SiC-Si/SiC | IMP | 1500 | 1.74 % | [29] | |
| HfB2-SiC/SiC | ISR | 1500 | 753 | 0.487 % | [30] |
| HfB2-SiC-MoSi2-Si/SiC- | - | 1650 | 618 | 57.9 % | [32] |
| Si | |||||
| | 2000 | 0.3 % | [33] | ||
| | PIP | 1300 | 120 | 1.25 % | [38] |
| SiCw | CVD | 1500 | 72 | 0.5 % | [41] |
| SiCw-/ZrB2-ZrSiO4 | 1500 | 20 | 120.17 g/cm2 | [43] | |
| SiCw-SiC-MoSi2-ZrB2 | CVD | 1500 | 124 | 1.1mg/cm2 | [44] |
| ZrB2-SiCw-BSG/ZrB2 | - | 1500 | 1.5 | 0.44 % | [45] |
| -MoSi2-SiCw-BSG | |||||
| SiCw-HfB2-SiC-Si/SiC | - | 1500 | 468 | 0.88 % | [46] |
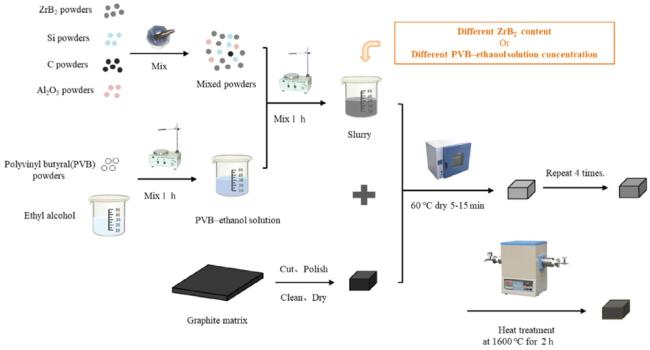
Fig. 10. Schematic diagram of the embedding process. Reproduced with permission from Ref. [52], © MPDI 2022. |
3.2. Brushing method
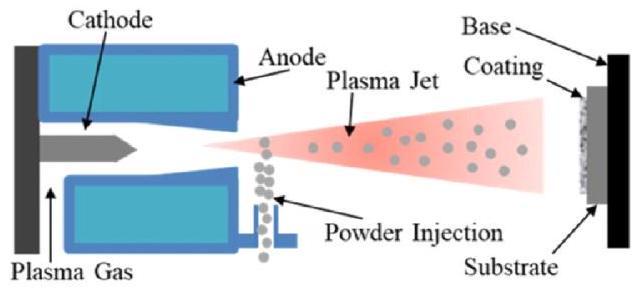
3.3. Spraying method
3.4. In-situ reaction method
Fig. 13. Schematic diagram of the in-situ reaction sintering process for preparing HfB2-MoSi2/SiC-Si coatings on the surface of C/C composites. Reproduced with permission from Ref. [73], © Elsevier 2020. |
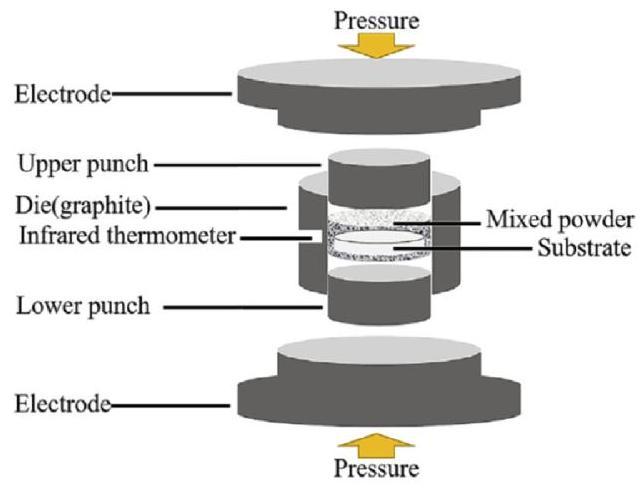
3.5. Spark plasma sintering
3.6. Other methods
4. Coating components
4.1. Boride composite coatings
4.2. Multi-component boride composite coatings
4.3. Boride-silicide composite coatings
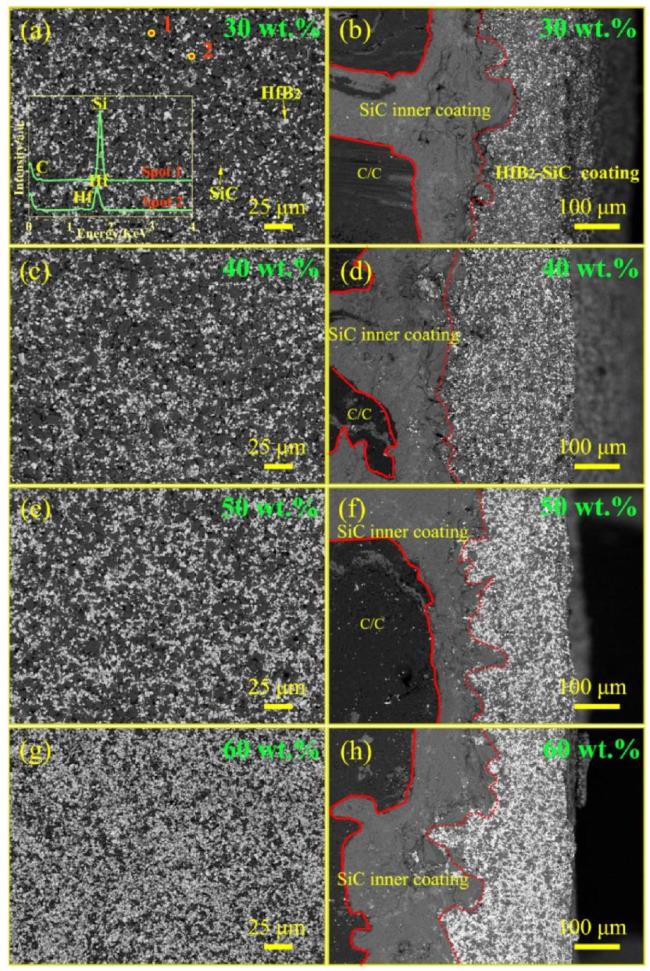
Fig. 16. Microstructure SEM images of HfB2-SiC coatings with different HfB2 contents: (a) and (b) Surface and cross-section of the 30wt\%HfB2-SiC coating; (c) and (d) Surface and cross-section of the 40wt\%HfB2-SiC coating; (e) and (f) Surface and cross-section of the 50wt\%HfB2-SiC coating; (g) and (h) Surface and crosssection of the 60wt\%HfB2-SiC coating. Reproduced with permission from Ref. [93], © Elsevier 2022. |
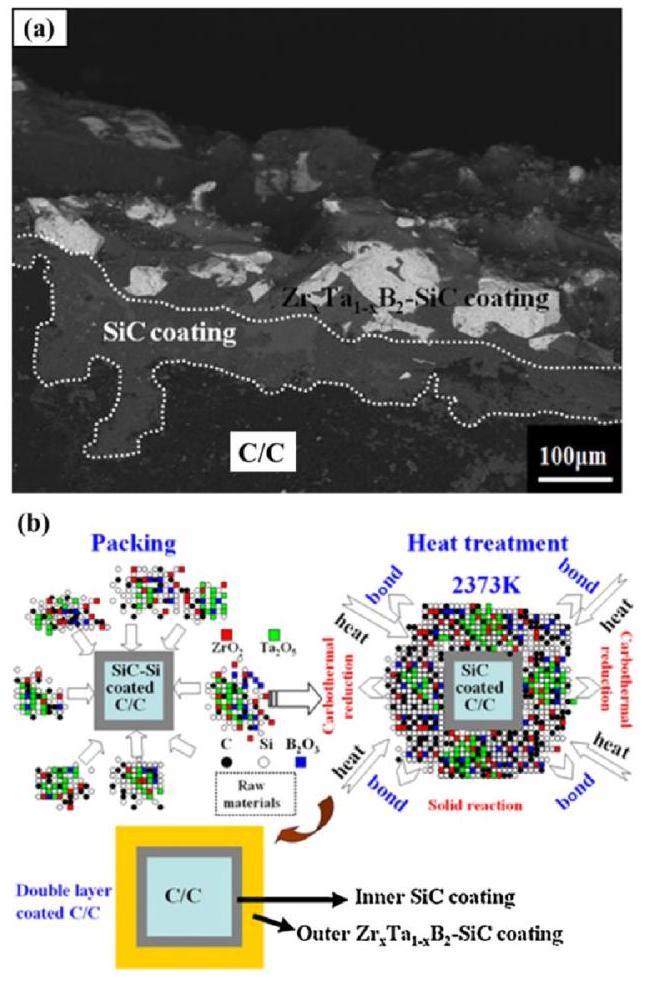
Fig. 17. w(a) Backscattered electron image of the cross-section of the double-layer coating on C/C composites; (b) Schematic of the preparation of the ZrxTa1-xB2-SiC outer coating. Reproduced with permission from Ref. [97], © Elsevier 2015. |
4.4. Boride composite coatings modified with rare earth oxides
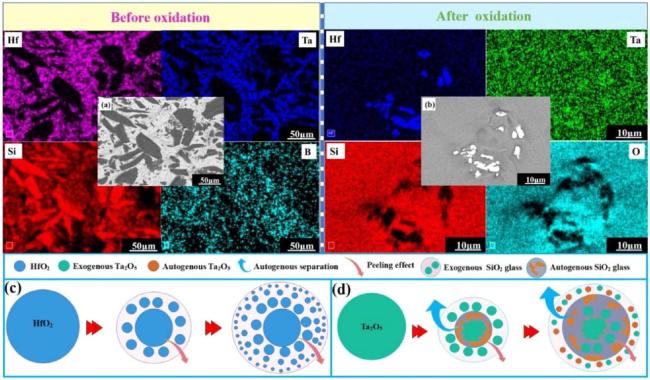
Fig. 18. (a)-(b) Surface EDS images of the 40HfB2-40TaSi2-20SiC coating samples before and after oxidation; (c)-(d) Schematic of Hf/Ta oxide peeling after oxidation. Reproduced with permission from Ref. [106], © Elsevier 2020. |
Fig. 19. Surface, cross-sectional morphology, and corresponding EDS analysis of ZS and ZSL coating samples after 550 h of oxidation at 1500∘C : (a) and (b) Secondary electron images of the surface of the ZS coating sample; (c) Backscattered electron image of the cross-section of the ZS coating sample; (d) and (e) Secondary electron images of the surface of the ZSL coating sample; (f) Backscattered electron image of the cross-section of the ZSL coating sample; (g) EDS area scan images of different elements corresponding to the cross-sectional morphology of the ZSL coating. Reproduced with permission from Ref. [110], © Elsevier 2018. |
5. Design of the self-generated glass film on the surface of coating
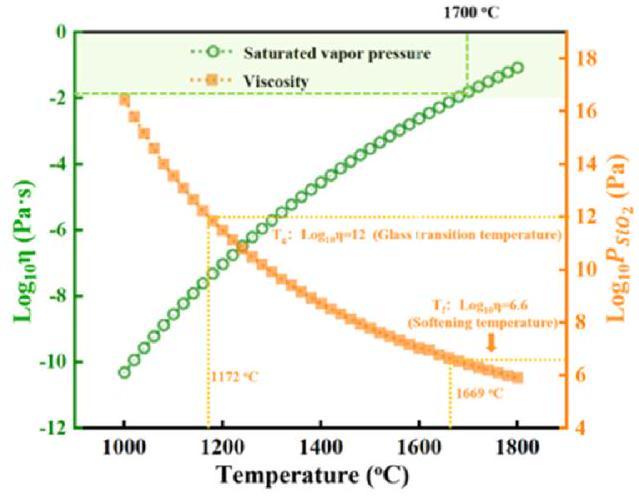
Fig. 20. Saturated vapor pressure and viscosity of SiO2 as a function of temperature. Reproduced with permission from Ref. [112], © Elsevier 2025. |
5.1. Transition metal-modified silicon-based glass film
5.2. Multi-transition-metal-modified silicon-based glass film
Fig. 23. TEM images of the composite glass layers: (a) Hf-B-Si-O and (d) Hf-Ta-B-Si-O; High-resolution TEM images of the composite glass layers: (b)-(c) Hf-B-Si-O and (e)-(f) Hf-Ta-B-Si-O. Reproduced with permission from Ref. [106], © Elsevier 2020. |
5.3. Synergistic modification of transition metals and rare earth elements
Fig. 24. (a)-(d) High-magnification backscattered SEM morphologies of the surface of the HfB2-SiC-LaB6 coating after oxidation at 1700∘C; (e) Evolution mechanism of the multiphase glass layer during the oxidation process. Reproduced with permission from Ref. [137], © Elsevier 2024. |
Table 2 The self-generated glass film coating has high temperature oxidation resistance above 1500∘C. |
| Coating materials | Fabrication methods | Temperature ( ∘C ) | Time (h) | Mass loss (wt%) | Refs. |
|---|---|---|---|---|---|
| HfB2-SiC/SiC | PCP | 1700 | 0.8 | 0.51 g/cm2 | [115] |
| HfB2-SiC-MoSi2 | LPS | 1500 | 200 | 0.08% | [121] |
| SiO2-MexOy | CP+HT | 1700 | 30 | - | [122] |
| ZrB2-SiC-ZrC-B4C | IMP + PYR | 1500 | 200 | 1.8% | [123] |
| ZrB2-SiC-ZrC | - | 1500 | 6 | 1.34 % | [124] |
| ZrB2-SiC-TaSi2 | SCC | 1700 | 0.5 | 3.81mg/cm2 | [126] |
| ZrB2-SiC-WB | LPPS | 1500 | 753 | 0.487 % | [127] |
| HfB2-SiC-MoSi2 | LFT | 1700 | - | 0.56 % | [128] |
| SiC/SiC-MoSi2-ZrB2 | 1500 | 30 | 0.3 % | [129] | |
| ZrB2-MoSi2-SiC-Si | SI+ VSI | 1600 | 150 | 0.21 % | [130] |
| ZrB2-xMoSi2-Y2O3-yAl | SCC | 1400 | 6.5 | [133] | |
| CeO2-HfB2-MoSi2-SiC | SPS | 1700 | 1.5 | 0.14 g/cm2 | [134] |
| ZrB2-SiC-La2O3/SiC | SPS | 1800 | 0.25 | 1.15mg/cm2 | [135] |
| Lu2O3-SiC-HfB2 | SPS | 1700 | 130 | 3.8mg/cm2 | [136] |
| LaB6-HfB2-SiC | LFT | 1700 | 1.5 | 0.85 g/cm2 | [137] |
6. Coating treatment
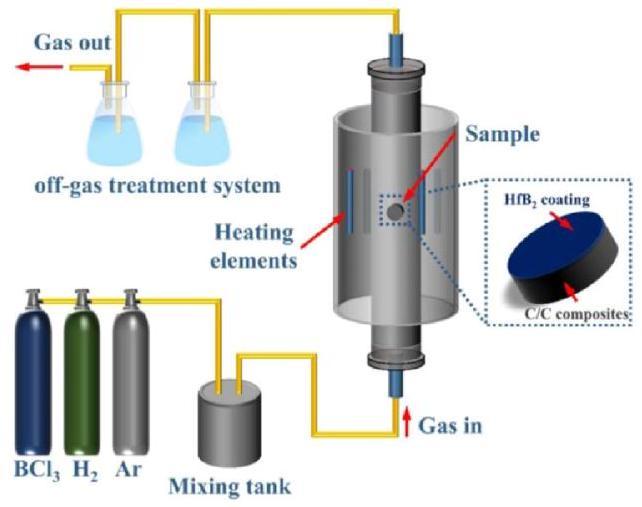
6.1. Chemical vapor deposition
Fig. 27. Schematic diagram of the preparation process for the SiC/ZrB2-CrSi2-Si/SiC multi-layer coating. Reproduced with permission from Ref. [148], © Elsevier 2021. |
6.2. Pre-oxidation
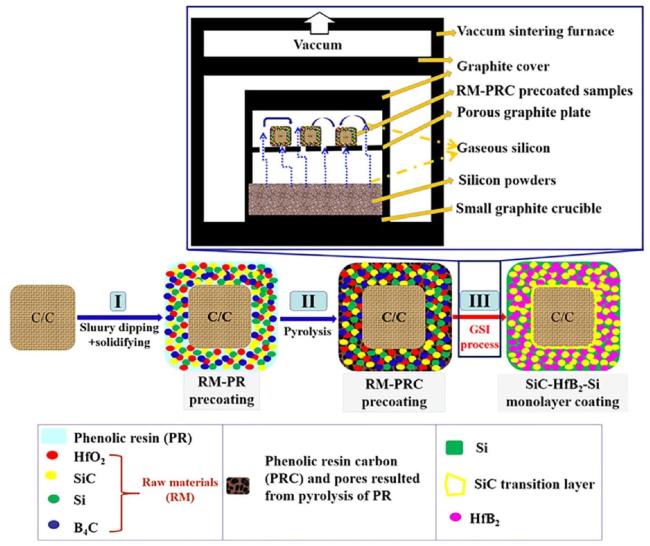
Fig. 28. Schematic diagram of the combined slurry dipping (SD) and gaseous silicon infiltration (GSI) process for preparing a single-layer SiC-HfB 2-Si coating on C/C composites. Reproduced with permission from Ref. [153], © Elsevier 2020. |
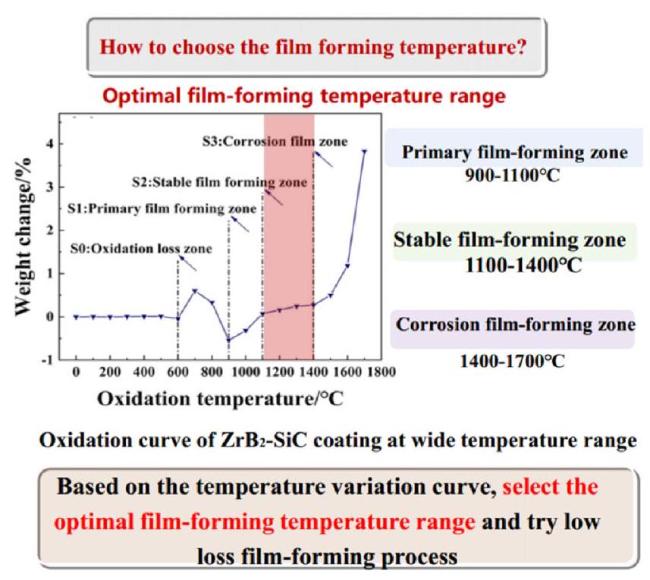
Fig. 29. Weight change curve of the ZrB2-SiC ceramic standard specimen during oxidation from room temperature to 1700∘C. Reproduced with permission from Ref. [155], © Elsevier 2022. |
6.3. Micro-zone repair
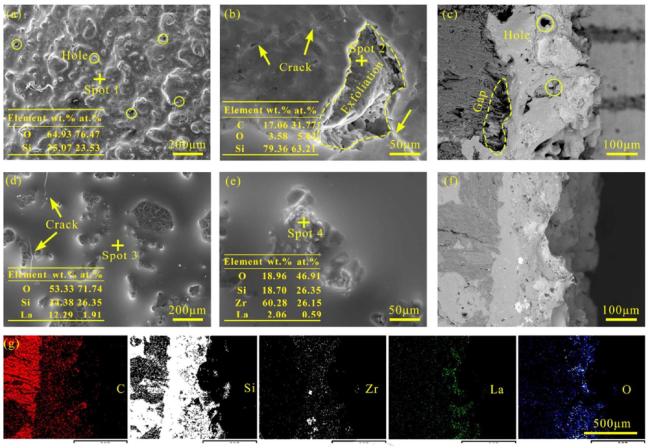
Fig. 30. (a) Surface SEM image and (b) TEM image of the ZrB2-SiC coating after 1200∘C,120 min film formation treatment; (c) EDS surface scan map corresponding to (a); (d) high-resolution TEM image corresponding to (b). Reproduced with permission from Ref. [155], © Elsevier 2022. |
Fig. 31. Schematic diagram of the coating repair process for C/C composites. Reproduced with permission from Ref. [162], © Elsevier 2022. |
7. Evaluation methods
7.1. Static oxidation
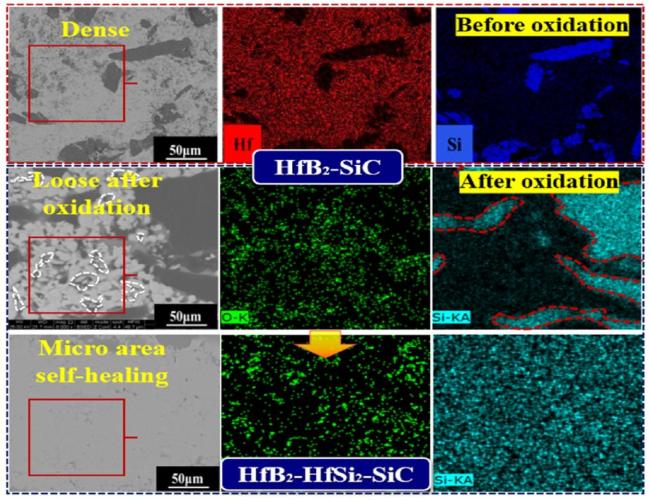
Fig. 32. In-situ micro-area self-healing during oxidation of HfB2-HfSi2-SiC coating prepared by SHS +SPS. Reproduced with permission from Ref. [165], © Elsevier 2023. |
7.1.1. Air oxidation
Fig. 33. Isothermal oxidation curve of the coated C/C composite in air at 1773 K. Reproduced with permission from Ref. [167], © Elsevier 2014. |
7.1.2. Water-oxygen environment
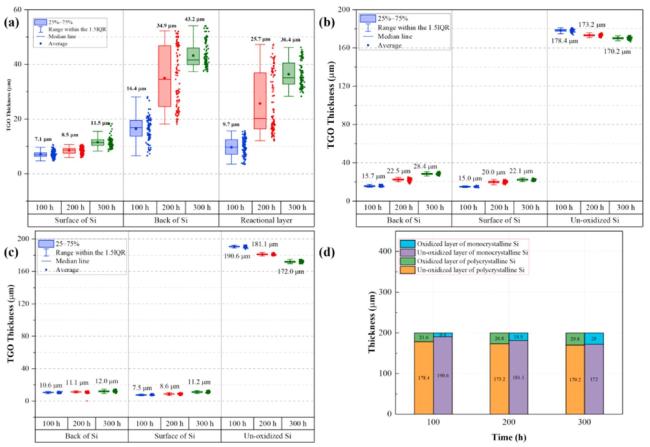
Fig. 34. Thickness measurements of thermally grown oxide (TGO) layers on three Si specimens after water vapor corrosion at 1300∘C : (a) APS-deposited Si coating; (b) polycrystalline Si; (c) single-crystal Si; (d) thickness comparison between unoxidized and oxidized crystalline Si. Reproduced with permission from Ref. [175], © Elsevier 2025. |
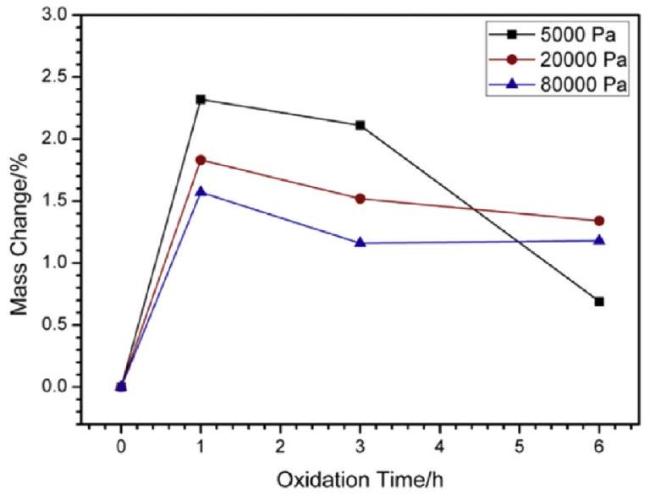
Fig. 35. Oxidation curves of coated samples at 1773 K under different OPP conditions. Reproduced with permission from Ref. [124], © Elsevier BV 2016. |
7.1.3. Influence of oxygen partial pressure
7.2. Dynamic oxidation
7.2.1. Thermogravimetric analysis and wide-temperature-range oxidation
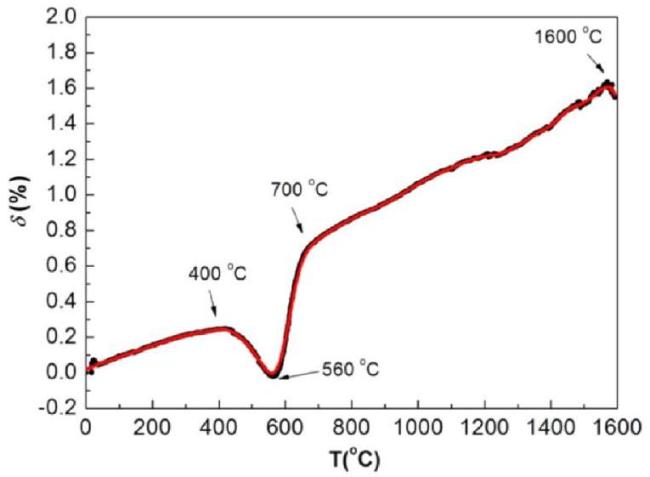
Fig. 36. Oxidation curve of SiC/ glaze precursor-coated samples in air from room temperature to 1600∘C. Reproduced with permission from Ref. [184], © Elsevier 2017. |
7.2.2. Thermal shock and cyclic oxidation
7.2.3. Wind tunnel testing
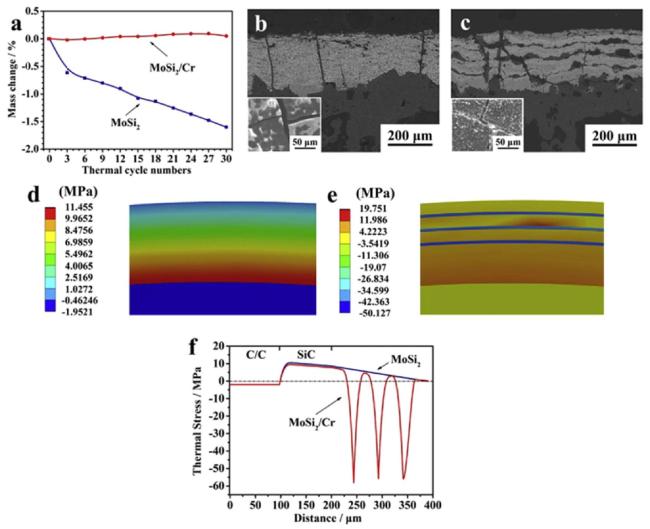
Fig. 37. Experimental and simulation results under thermal shock environment: (a) mass change curves of the coated samples with monolayer MoSi 2 coating and with laminated MoSi2/Cr coating after 30 thermal cycles; (b) BSE image of monolayer MoSi2 coating (inset of figure is the surface view of (b)) and (c) BSE image of laminated MoSi2/Cr coating along the cross-section (inset of figure is the surface view of (c)) after thermal shock test; (d) and (e) stress distribution diagram along the cross-section of monolayer MoSi2 coating and laminated MoSi2/Cr coating during a thermal cycle; (f) stress distribution curves through the thickness of the coating and substrate under thermal shock environment. Reproduced with permission from Ref. [190], © Elsevier 2020. |
7.3. High oxygen-blocking evaluation
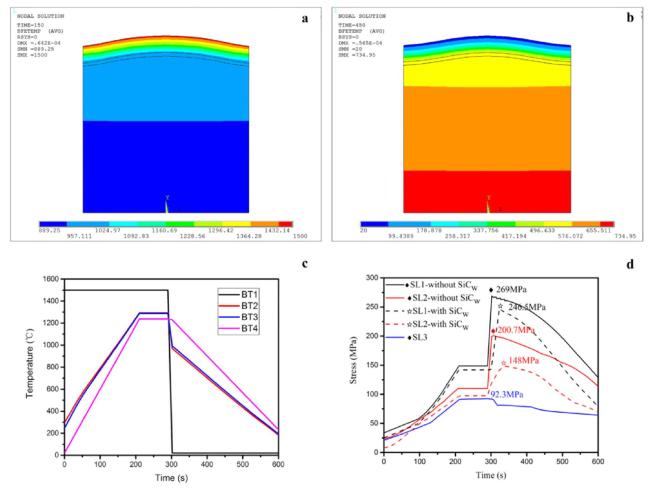
Fig. 38. Temperature and thermal stress distribution in coated C/C composites. Reproduced with permission from Ref. [46], © Elsevier 2018. |
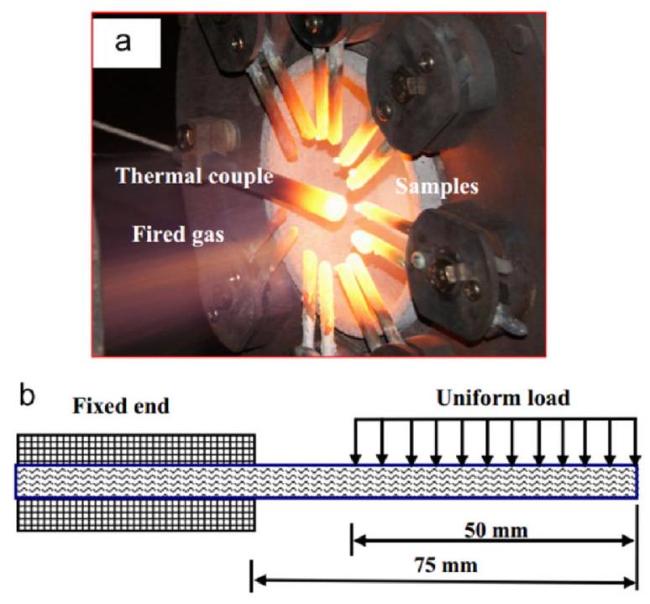
Fig. 39. Macroscopic images and cantilever beam model of coated samples in wind tunnel. Reproduced with permission from Ref. [199], © Elsevier 2015. |
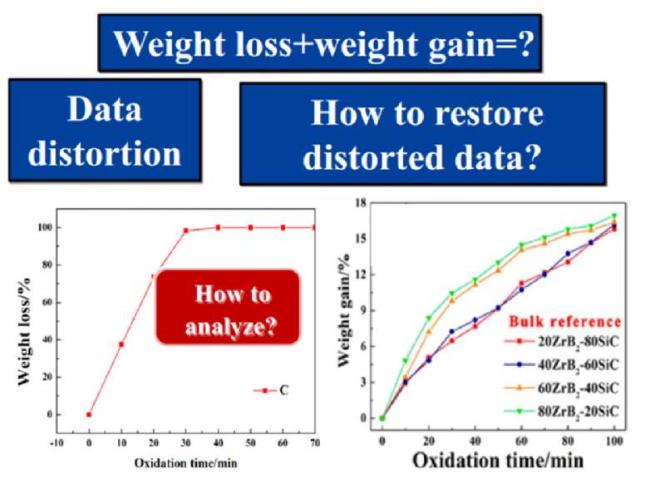
Fig. 40. Data distortion in traditional evaluation methods. Reproduced with permission from Ref. [143], © Elsevier 2021. |
Fig. 41. High oxygen-barrier evaluation system. Reproduced with permission from Ref. [143], © Elsevier 2021. |