1. Introduction
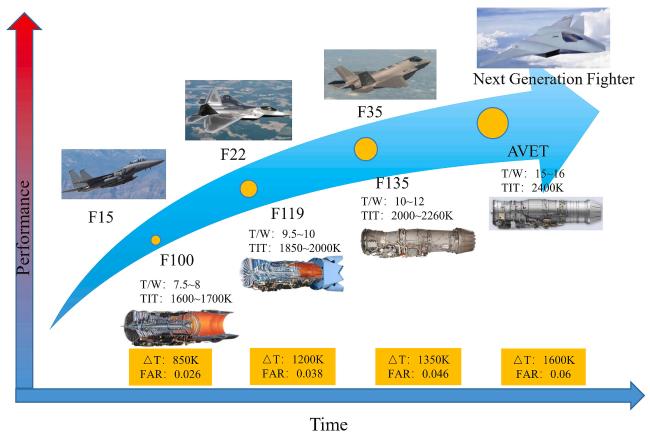
Fig. 1 Development trend of the engine and its combustor. Reproduced with permission from Ref. [5], © Elsevier 2023. |
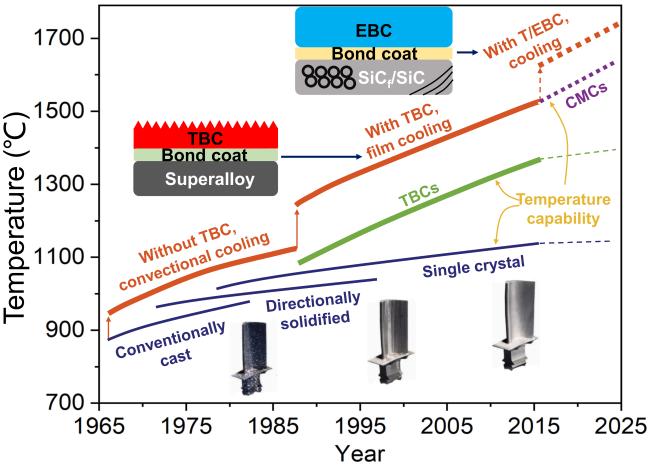
Fig. 2 The progression and projection of temperature capabilities of Ni-based superalloy, TBC, EBC and CMC gas-turbine engine materials, and maximum allowable gas temperatures with cooling [6]. |
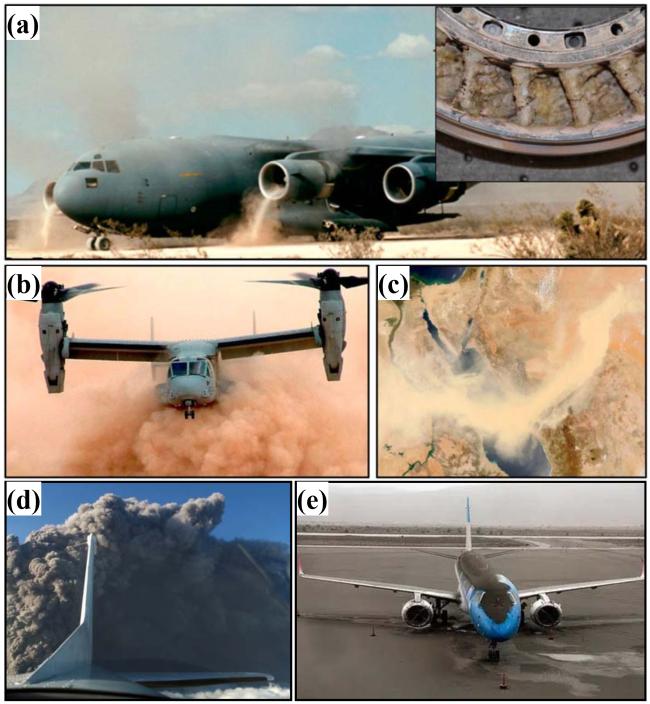
Fig. 3 (a) C-17 military aircraft ingesting sand during take-off on unimproved runway Inset: Gas turbine engine vanes with molten CMAS deposits, (b) NAVY V-22 rotorcraft performing landing maneuver under severe ‘brown-out’ heavy dust/sand conditions, (c) Dust storm across the Red Sea May 13, 2005, (d) A plane passing through a cloud of volcanic ash, and (e) Volcanic ash deposited on aircraft. Reproduced with permission from Ref. [18], © Sage 2021. |
Fig. 4 Military aircraft flying during a sand storm in an arid region. Image insert—mineral dust particles passing through the gas-turbine and corroding the ceramic coatings and metallic surfaces of the hot section components. Reproduced with permission from Ref. [21], © Wiley Online Library 2019. |
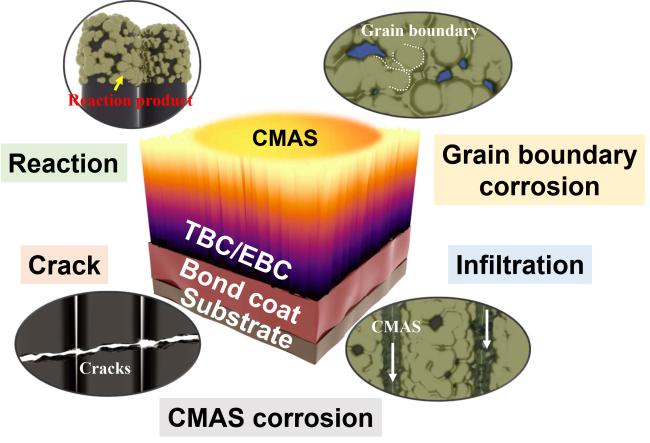
Fig. 5 Schematic diagram of failures caused by CMAS on TBCs and EBCs. |
2. Historical overview of CMAS corrosion and characteristics of CMAS
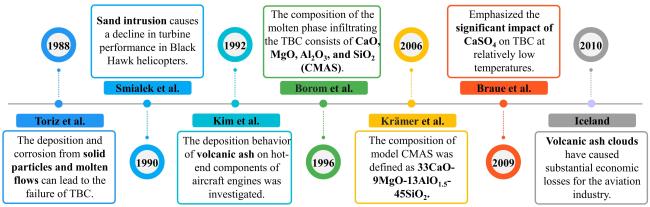
2.1. Historical overview of CMAS corrosion
2.2. Characteristics of CMAS
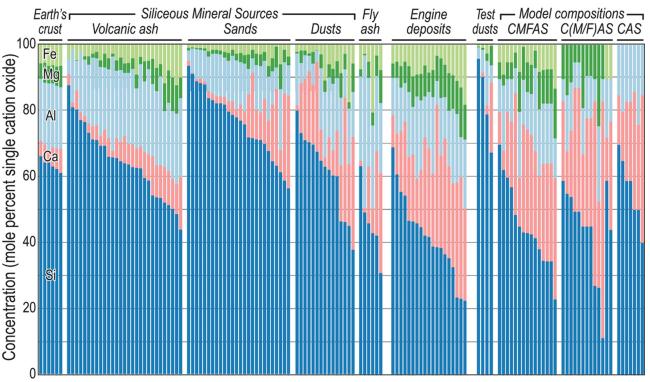
Fig. 7 The composition of CMAS from different sources. Reproduced with permission from Ref. [33], © Wiley Online Library 2022. |
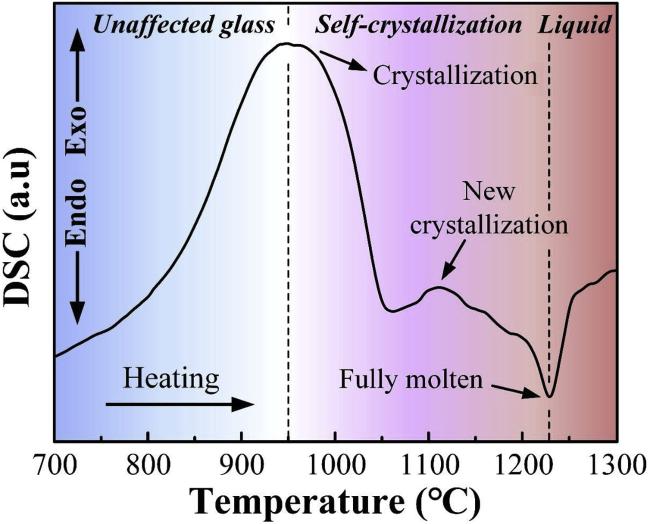
Fig. 8 The DSC curve of CMAS with a composition of 33CaO-9MgO-13AlO1.5-45SiO2 . Reproduced with permission from Ref. [35], © Elsevier 2020. |
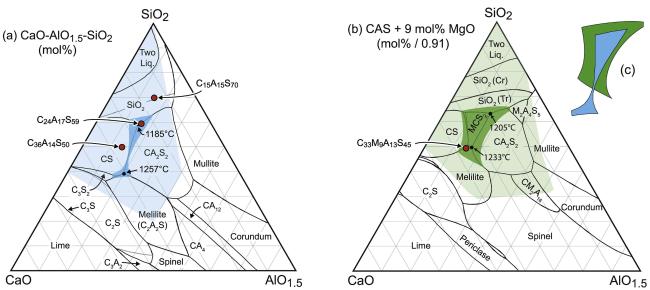
Fig. 9 (a) Phase diagram of the CaO-MgO-AlO1.5 ternary system at 1300 ℃; (b) Phase diagram of the CaO-MgO-AlO1.5 ternary system with the addition of 9 mol% MgO; (c) The effect of MgO addition on the shape and extent of the liquid field. Reproduced with permission from Ref. [36], © Elsevier 2016. |
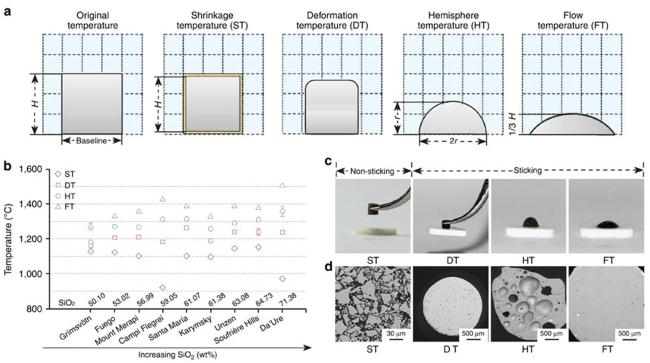
Fig. 10 (a) Schematic diagram of the volcanic ash melting process; (b) Transition temperatures of volcanic ash with different compositions at various stages; (c) Photographs of volcanic ash at different melting stages; (d) Backscattered electron images of volcanic ash at various melting stages. Reproduced with permission from Ref. [37], ©The Author(s) 2016. |
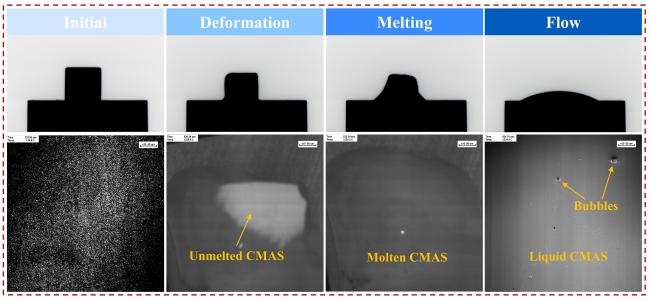
Fig. 11 In-situ observation of the melting process of CMAS. |
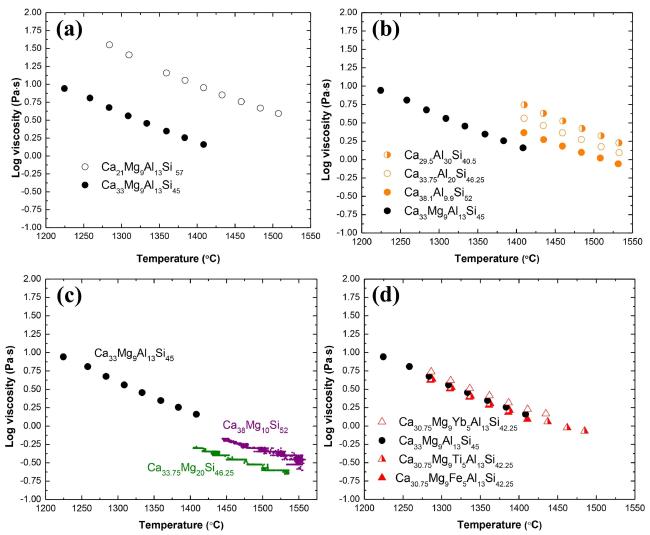
Fig. 12 The relationship between viscosity and temperature for CMAS with different compositions. (a) Different CaO/SiO2 ratios; (b) Different AlO1.5 contents; (c) Different MgO contents; (d) Addition of other oxides. Reproduced with permission from Ref. [38], © Elsevier 2022. |
3. Interaction between CMAS and TBCs at high temperatures
3.1. CMAS corrosion mechanism of ZrO2-based TBC
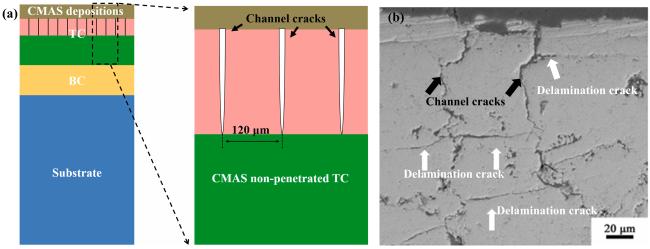
Fig. 14. (a) Schematic diagram of CMAS-penetrated TBC model with channel cracks and (b) channel and delamination cracks of YSZ on cross-section. Reproduced with permission from Ref. [56], © Elsvier 2023. |
Fig. 15. (a) The columnar morphology and cracks of TBC in the nozzle guiding vane, (b) infiltration of CMAS in TBC, (c) crack formed due to merging of cooling holes and (d) choking of cooling hole. Reproduced with permission from Ref. [59], © Autuor(s) 2018. |
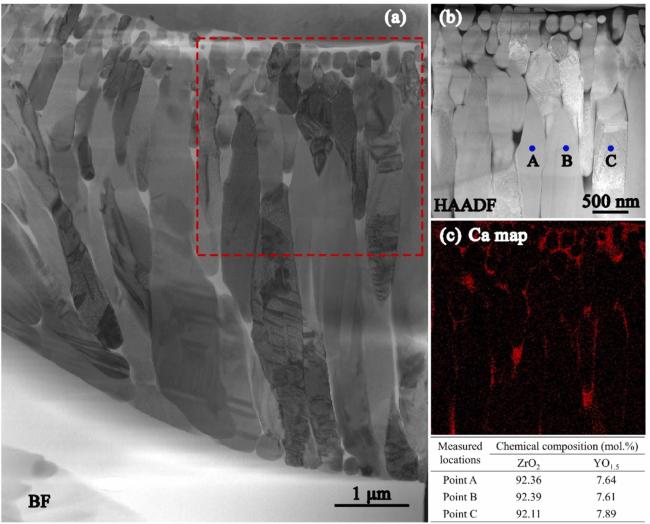
Fig 16. (a) Bright-field TEM image of CMAS infiltration front at 1250 ℃ for 2 h in 8YSZ TBC, (b-c) HAADF image, and the corresponding Ca mapping of the dashed rectangle area in (a). The table at the bottom right lists the chemical compositions of points A, B, and C in (b). Reproduced with permission from Ref. [60], © Elsvier 2022. |
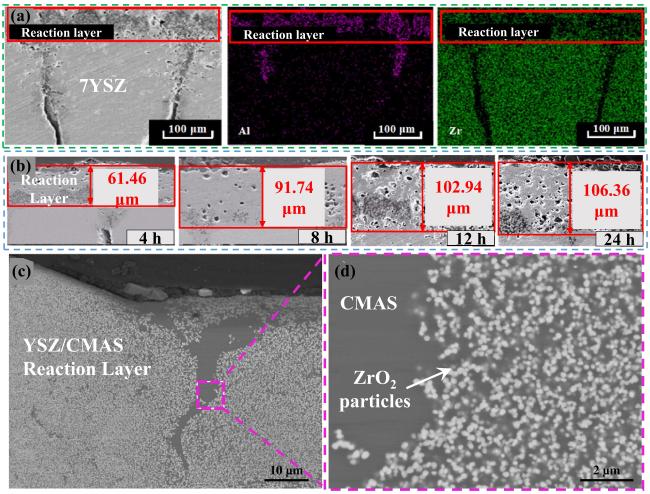
Fig. 17. (a) SEM and EDS images of the interaction layer on cross-section, (b) the thickness of the reaction layer at different holding times, and (c-d) cross-section of the CMAS/YSZ interaction zone in the dissolution/reprecipitation process. Reproduced with permission from Ref. [61] for (a, b), © Author(s) 2021. Ref. [64] for (c, d), © Elsvier 2013. |
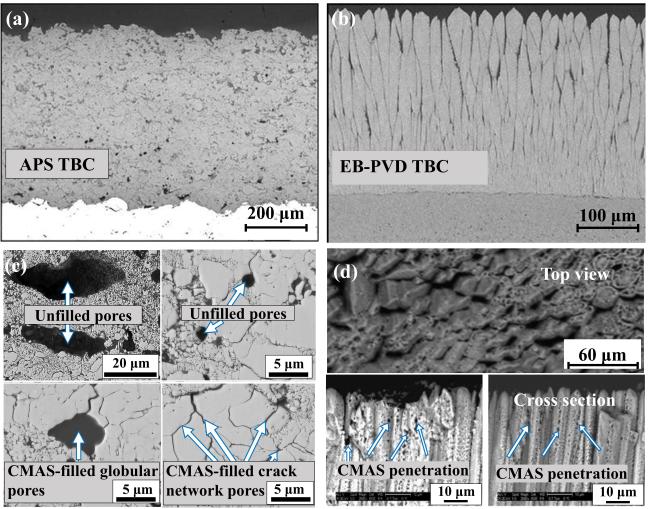
Fig. 18. Typical microstructure and the microstructure after CMAS of TBC prepared by APS and EB-PVD: (a) 7YSZ TBC prepared by APS and (b) EB-PVD, (c) microstructures of 8YSZ APS TBC after CMAS corrosion at 1250 ℃ for 3 h and (d) microstructures of 7YSZ EB-PVD TBC after CMAS corrosion at 1300 ℃ for 4 h. Reproduced with permission from Ref. [65] for (a, b), © Springer Nature 2008. Ref. [66] for (c), © Elsvier 2020. Ref. [67] for (d), © Elsvier 2010. |
3.2. CMAS corrosion mechanism of RE2Zr2O7
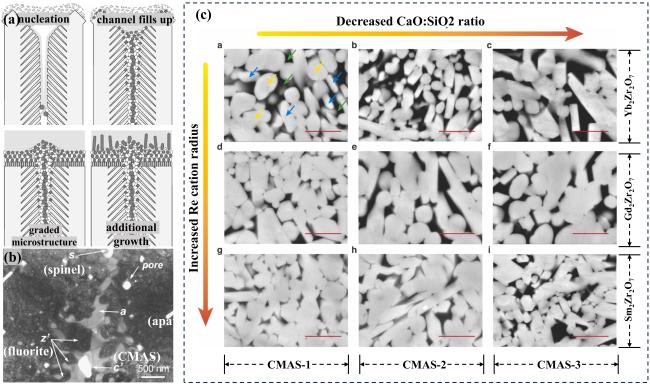
Fig. 19. (a) Schematic representation of the envisaged mechanism leading to the microstructure of the RE2Zr2O7 reaction layer, (b) bright field TEM micrograph of the corrosion reaction zone of the RE2Zr2O7. Phases: z’: fluorite, a: apatite, s: spinel, p: pore, c’: CMAS, (c) Back-scattering SEM images demonstrating the crystallization products in different RE2Zr2O7-CMAS systems from high-temperature reaction experiments conducted at 1300 ℃/30 min. Reproduced with permission from Ref. [70] for (a, b), © John Wiley & Sons - Books 2008. Ref. [72] for (c), © Elsvier 2023. |
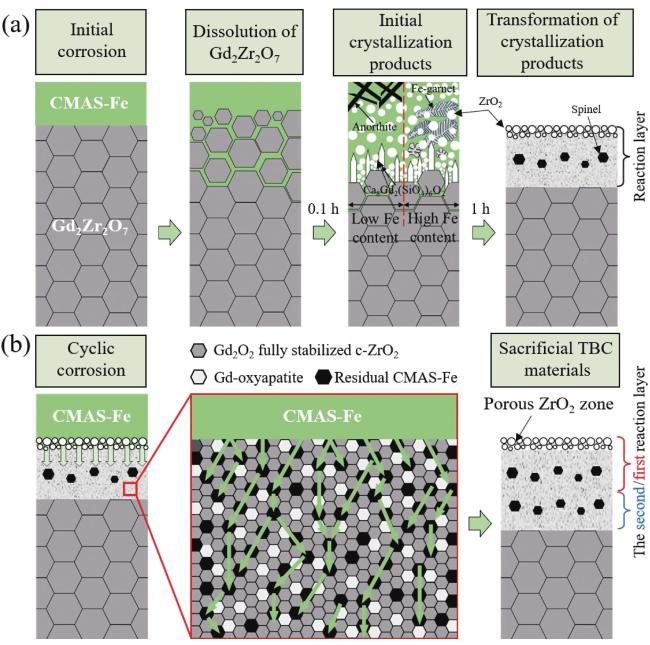
Fig. 20. Schematic diagram of formation and growth of reaction layer of Gd2Zr2O7 coating under CMAS-Fe corrosion. Reproduced with permission from Ref. [73], © Author(s) 2024. |
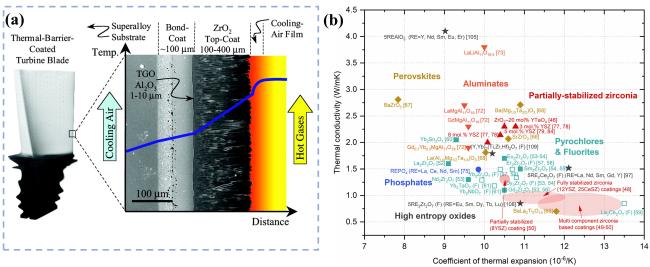
Table 1 Thermal conductivity and thermal expansion coefficient of RE2Zr2O7 [68] |
| Compound | Thermal conductivity (W·m-1·K-1) | Thermal expansion coefficient (10-6·K-1) |
|---|---|---|
| La2Zr2O7 | 1.60(700 °C) | (8~9)×10-6(RT~1000 °C) |
| Nd2Zr2O7 | 1.60(700 °C) | (9~9.5)×10-6(RT~1200 °C) |
| Sm2Zr2O7 | 1.50(700 °C) | 10.9×10-6(RT~1000 °C) |
| Gd2Zr2O7 | 1.40(800 °C) | 11.5×10-6(1000 °C) |
| Dy2Zr2O7 | 1.34(800 °C) | (8~11)×10-6(RT~1000 °C) |
| Er2Zr2O7 | 1.50(800 °C) | (7.7~11)×10-6(RT~1200 °C) |
| Yb2Zr2O7 | 1.40(800 °C) | (7.6~10.7)×10-6(RT~1200 °C) |
| 8YSZ | 2.30(800 °C) | (10~11)×10-6(RT~1000 °C) |
3.3. CMAS corrosion mechanism of RETaO4
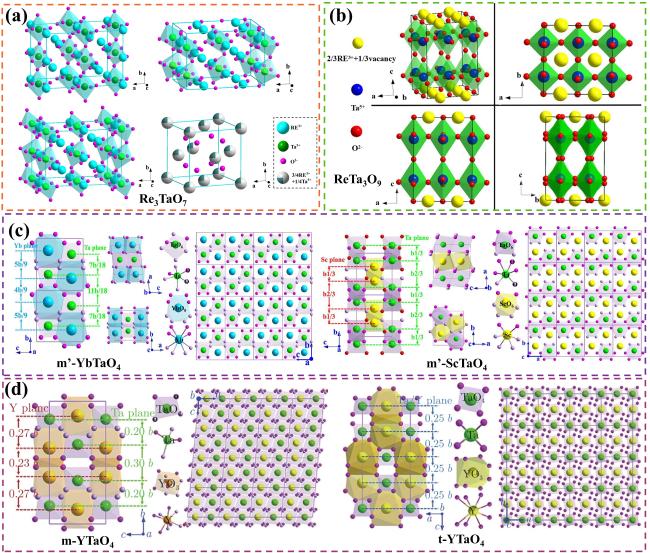
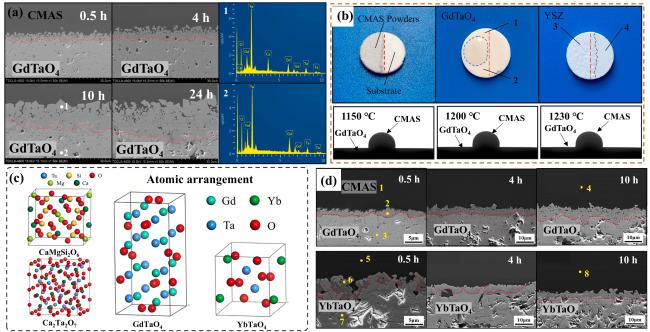
Fig. 22. The interface microstructure, wetting process on-line photographs and atomic arrangement of GdTaO4 and YbTaO4: (a) the interface microstructure of GdTaO4 and CMAS (33Ca-9Mg-13Al-45Si) at 1350 ℃ for different time, (b) the surface state of GdTaO4 and YSZ before and after high-temperature reaction, (c) the atomic arrangement of CaMgSi2O6, Ca2Ta2O7, GdTaO4 and YbTaO4 and (d) images of the reaction interface between residual CMAS and substrate. Reproduced with permission from Ref. [81] for (a, b), © Elsevier 2021. Ref. [82] for (c, d), © Elsevier 2022. |
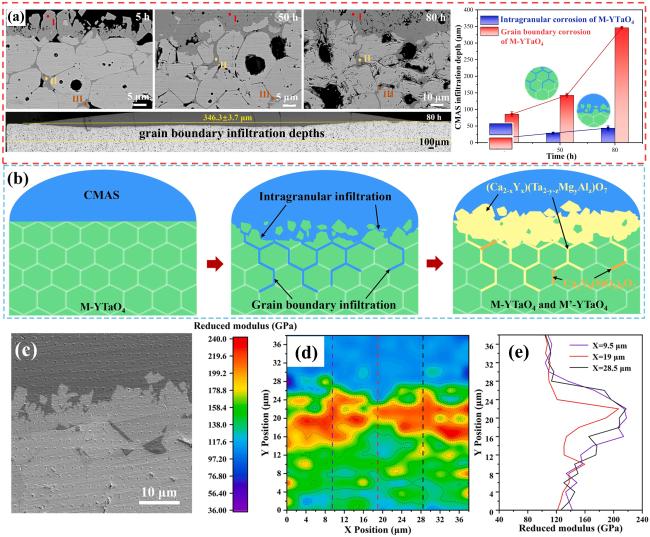
Fig. 23. Corrosion mechanism of M-YTaO4: (a) images of cross-sections and grain boundary infiltration depths of M-YTaO4 after CMAS corrosion, (b) schematic diagram of CMAS corrosion of M-YTaO4, (c) the interface between residual CMAS melt and M-YTaO4 substrate after CMAS corrosion at 1300 ℃ for 5 h, (d) Mapping and (e) section lines of reduced moduli corresponding to (c). Reproduced with permission from Ref. [83], © Elsevier 2024. |
3.4 CMAS corrosion mechanism of REPO4
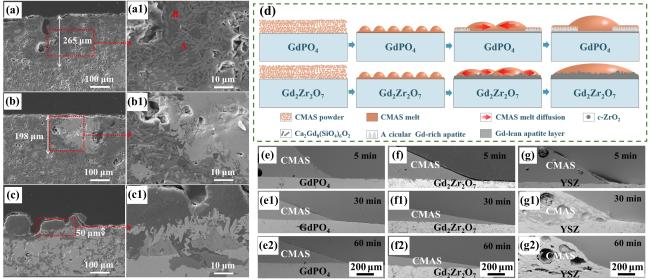
Fig. 24. (a-c) SEM images of the cross-sectional morphologies of LMA/GdPO4 after CMAS corrosion, (d) Schematic diagrams of CMAS wetting behavior of GdPO4 and Gd2Zr2O7, and cross-sectional images of contact angles of CMAS droplets on (e-e2) GdPO4, (f-f2) Gd2Zr2O7, and (g-g2) YSZ. Reproduced with permission from Ref. [85] for (a-c), © Elsevier 2023. Ref. [86] for (d-g), © Author(s) 2024. |
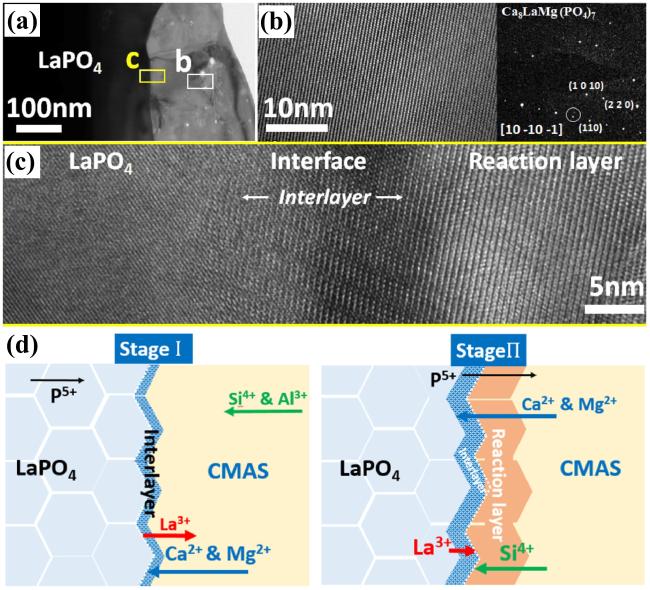
Fig. 25. (a) Bright-field TEM image of LaPO4 and its reaction layer with CMAS after CMAS attack at 1250 ℃ for 5min, (b) HRTEM image of the reaction layer in (a), with an inset image of the diffraction pattern of the reaction layer, (c) HRTEM image of interlayer, and (d) schematic of the transport mechanisms associated with the formation of a transient interlayer and reaction layer across the LaPO4-CMAS interface when exposed to CMAS attack at 1250 ℃. Reproduced with permission from Ref. [87], © Author(s) 2024. |
4. Degradation of EBCs caused by CMAS at high temperatures
4.1. CMAS corrosion behavior of rare earth monosilicate (RE2SiO5)
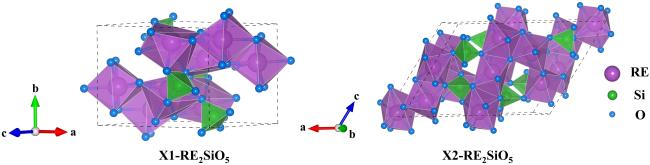
Fig. 27 The crystal structures of RE2SiO5. |
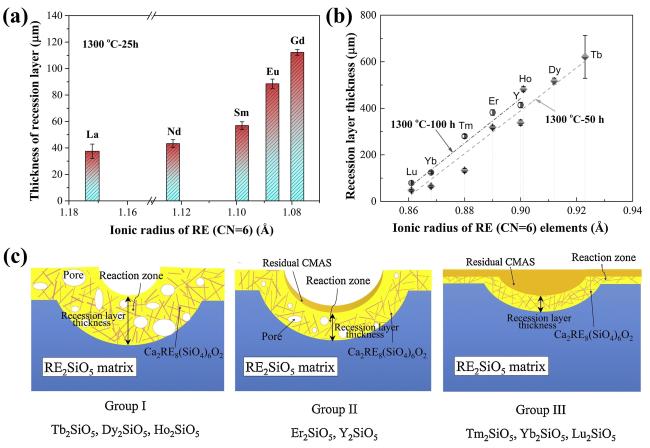
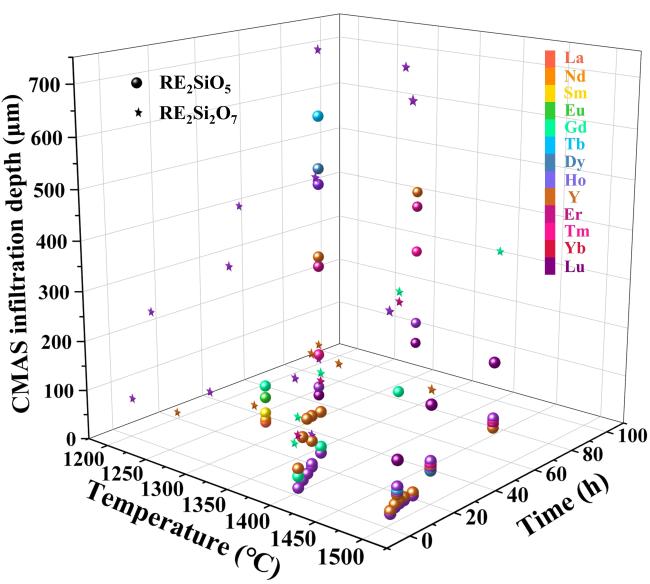
Fig. 28 (a) The recession layer thickness of X1-RE2SiO5 (RE = La, Nd, Sm, Eu, Gd) after CMAS corrosion at 1300 ℃ for 25 h; (b) The recession layer thickness of X2- RE2SiO5 (RE = Tb, Dy, Ho, Er, Y, Tm, Yb, and Lu) after CMAS corrosion at 1300 ℃for 50 h and 100 h; (c) Schematic diagram of CMAS corrosion of X2-RE2SiO5 at 1300 ℃. Reproduced with permission from Ref. [101] for (a), © Elsevier 2019. Reproduced with permission from Ref. [106] for (b, c), © Elsevier 2019. |
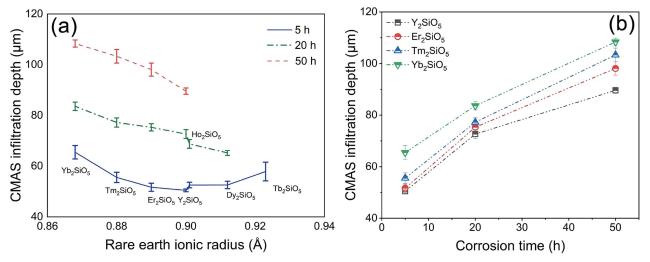
Fig. 30 The CMAS infiltration depth of X2-RE2SiO5 (RE = Tb, Dy, Ho, Er, Y, Tm, and Yb) after CMAS corrosion at 1500 ℃. Reproduced with permission from Ref. [110], © The Author(s) 2023. |
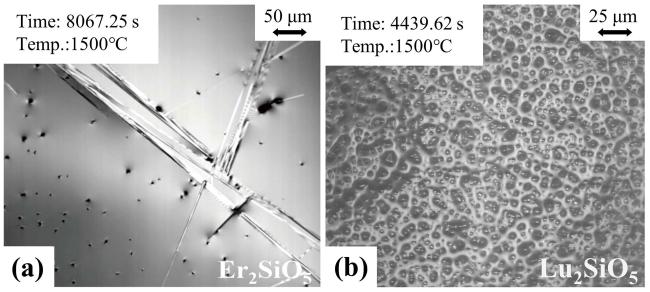
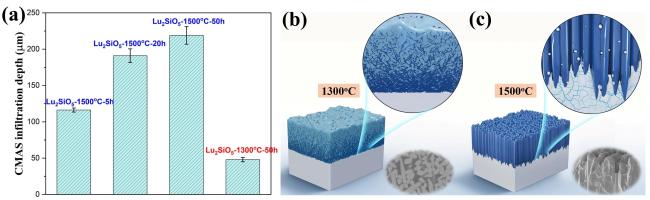
Fig. 31 (a) CMAS infiltration depth of Lu2SiO5 at 1300 ℃ and 1500 ℃. Schematic diagram of the CMAS corrosion behavior of Lu2SiO5 at (b) 1300 ℃ and (c) 1500 ℃. Reproduced with permission from Ref. [109], © Elsevier 2024. |
4.2. CMAS corrosion behavior of rare earth disilicate (RE2Si2O7)
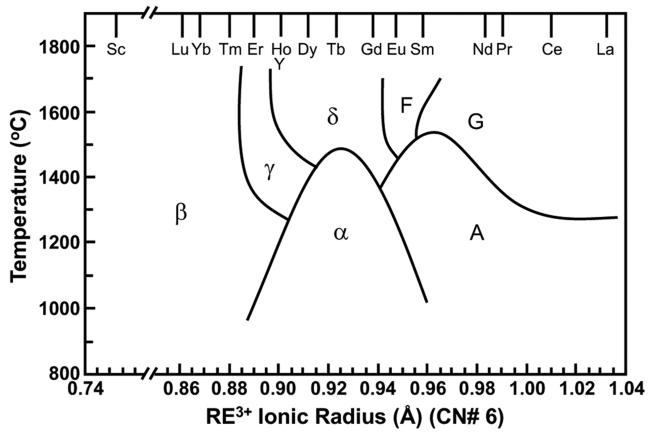
Fig. 32 The relationship between the polymorphism of RE2Si2O7 and the temperature as well as the RE ionic radius. Reproduced with permission from Ref. [100], © Elsevier 2018. |
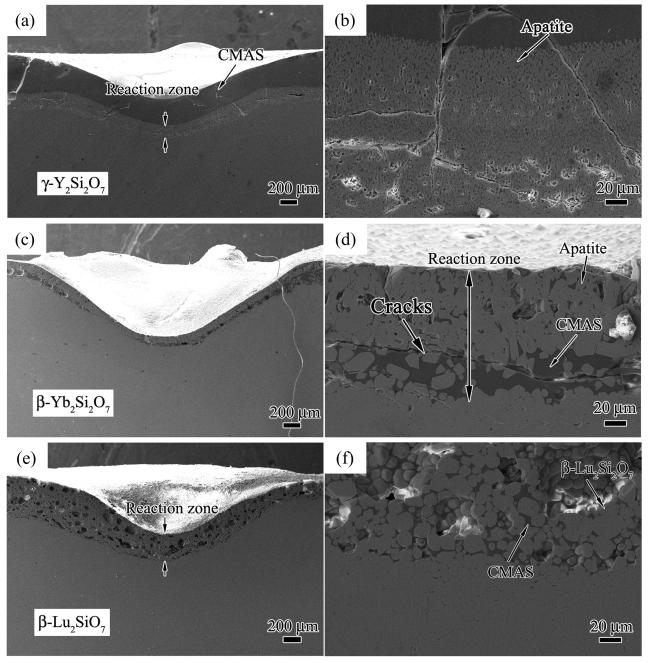
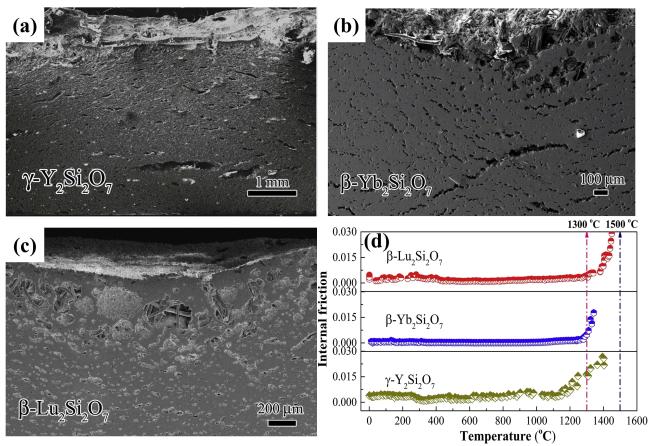
Fig. 33 Cross-sectional morphologies after CMAS corrosion at 1300 ℃: (a, b) γ-Y2Si2O7, (c, d) β-Yb2Si2O7, and (e, f) β-Lu2Si2O7. Reproduced with permission from Ref. [114], © Elsevier 2019. |
Fig. 34 (a-c) Cross-sectional morphology of RE2Si2O7 (RE = Y, Yb, and Lu) after CMAS corrosion at 1500 ℃; (d) Temperature-dependent internal friction of RE2Si2O7 (RE = Y, Yb, and Lu). Reproduced with permission from Ref. [114], © Elsevier 2019. |
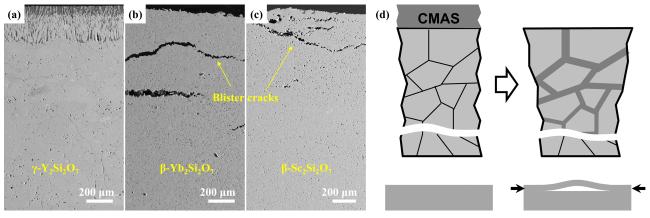
Fig. 36 (a-c) Cross-sectional morphology of γ-Y2Si2O7, β-Yb2Si2O7, and β-Sc2Si2O7 after CMAS corrosion at 1500 ℃; (d) Schematic diagram of the formation of “blister” cracks. Reproduced with permission from Ref. [116] for (a), © Elsevier 2018. Reproduced with permission from Ref. [115] for (b-d), © Elsevier 2018. |
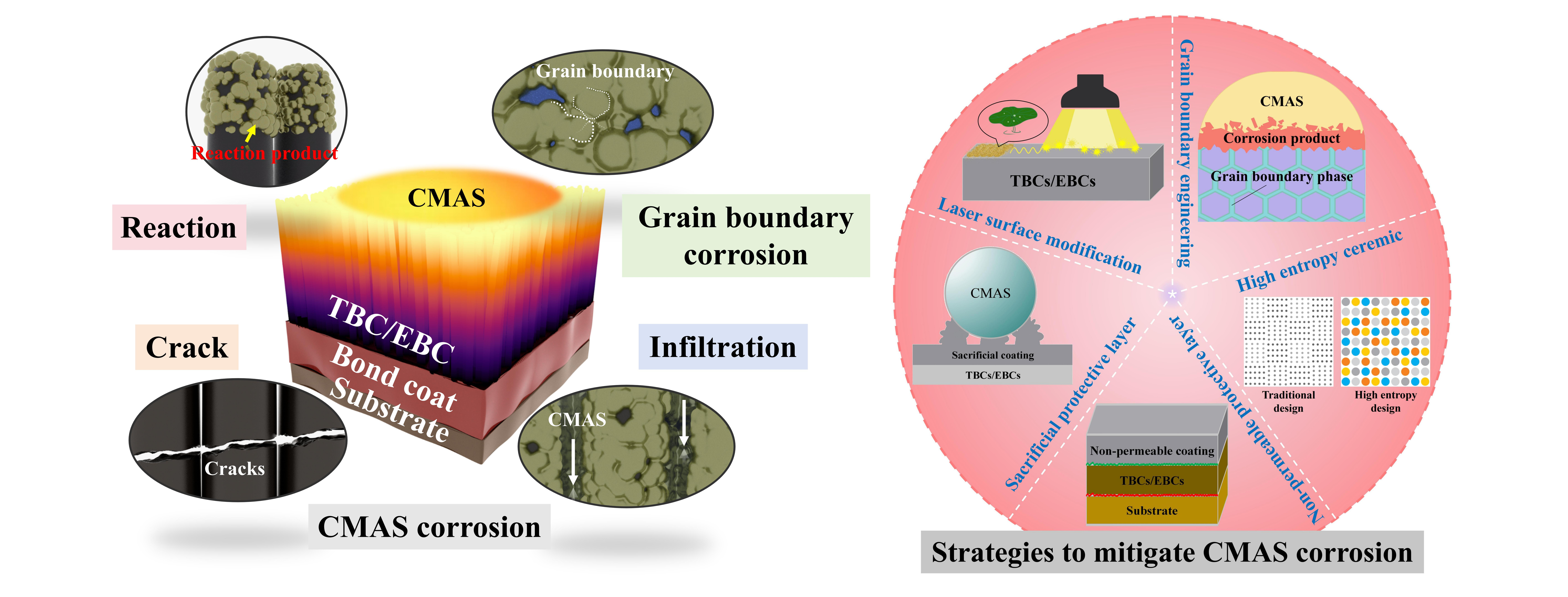
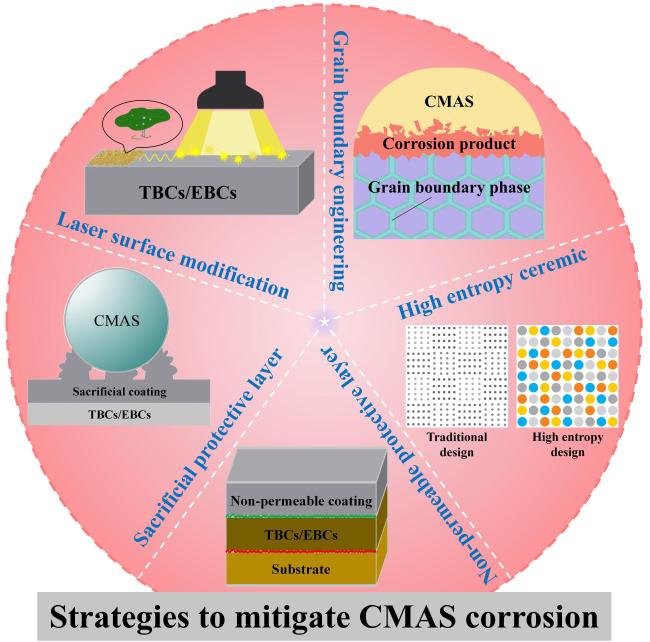
5. Strategies to mitigate CMAS corrosion
Fig. 37 Strategies to mitigate CMAS corrosion of TBCs and EBCs. |
5.1. Design the non-permeable protective coating
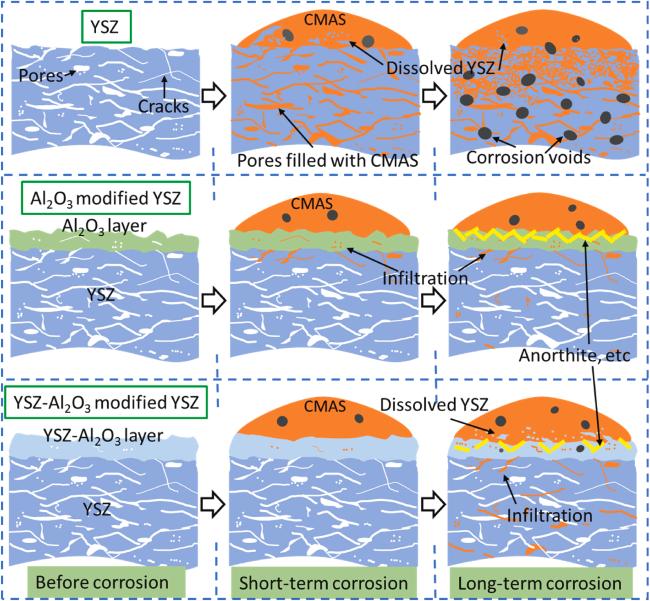
5.2. Design the sacrificial protective coating
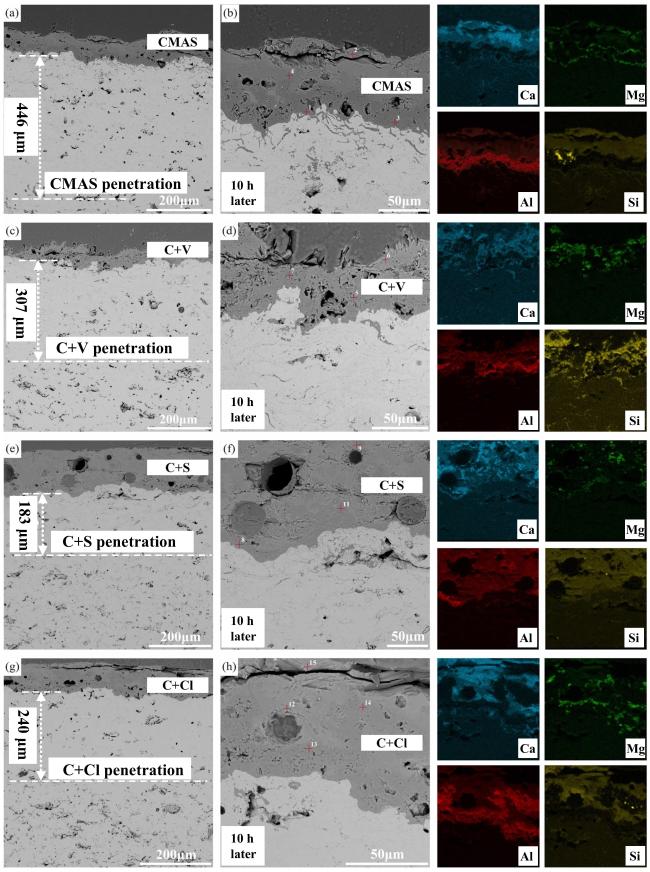
Fig. 39 A cross-sectional SEM images of the Al2O3-YSZ coating exposed to (a-b) CMAS, (c-d) CMAS+NaVO3 powders, (e-f) CMAS+Na2SO4 powders, and (g-h) CMAS+NaCl powders for 10 h, and corresponding EDS mapping results (Ca, Mg, Al, and Si elements) are also provided. Reproduced with permission from Ref. [131], © Elsevier 2024. |
5.3. Optimizing coating surface structure
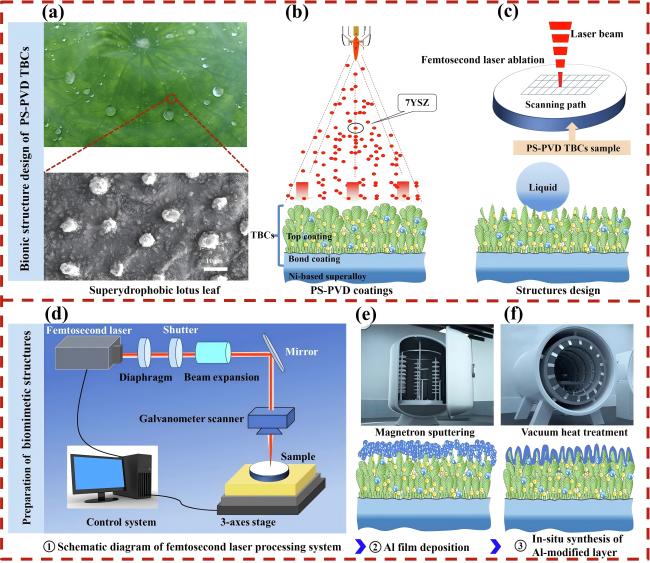
Fig. 40 (a) Superhydrophobic lotus leaf and its microstructure-the source of inspiration. (b) PS-PVD TBCs preparation. (c) Constructing the lotus leaf structure on PS-PVD TBCs surface. (d) Schematic diagram of femtosecond laser processing system and fabrication of micro-nanostructured PS-PVD TBCs. (e) Al film deposition by magnetron sputtering. (f) In situ synthesis of Al-modified layer on laser textured PS-PVD TBCs by vacuum heat treatment. Reproduced with permission from Ref. [132], ©The Author(s) 2024. |
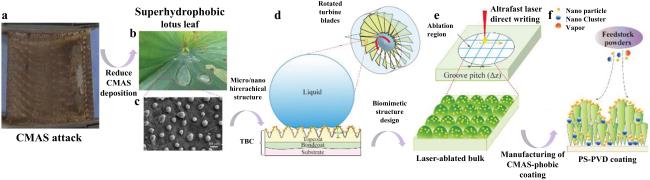
Fig. 41 (a) Volcanic ash accumulation on turbine blades leading to engine failure. (b) Lotus leaf-inspired superhydrophobicity for CMAS-phobic surface design. (c) Micro/nano hierarchical structure on TBC surface reducing CMAS deposition. (d) Biomimetic structure application on turbine blades to prevent CMAS adherence. (e) Ultrafast laser direct writing for micro/nano hierarchical structure on (Gd0.9Yb0.1)2Zr2O7 material. (f) PS-PVD process for CMAS-phobic coating with microconical papillae and nanoparticles. Reproduced with permission from Ref. [133], ©The Author(s) 2023. |
5.4. Enhancing CMAS corrosion resistance via grain boundary engineering
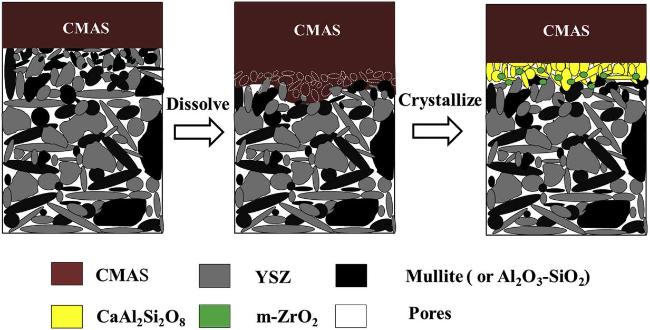
Fig. 42 The etching process of the ceramic material by CMAS. Left: CMAS initially erodes YSZ grains. Middle: CMAS dissolves YSZ to form CaAl2Si2O8 phase. On the right: CMAS crystallizes, forming the m-ZrO2 phase. Reproduced with permission from Ref. [138], ©The Author(s) 2019. |
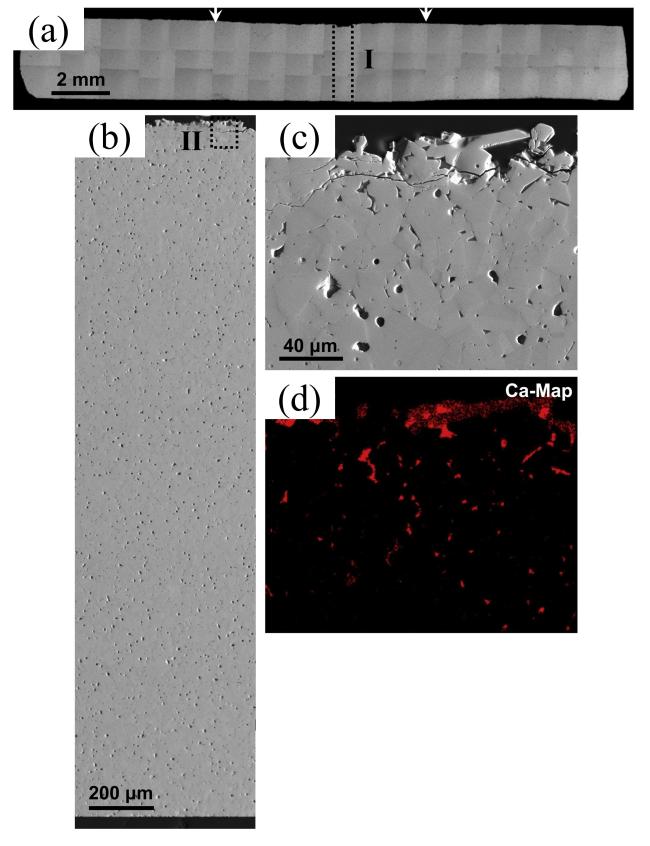
Fig. 43 (a) Collage of cross-sectional optical micrographs of β-Yb2Si2O7/1 vol% CMAS pellet that have interacted with CMAS at 1500 ℃ for 24 h. The region between the arrows is where the CMAS was applied. (b) Cross-sectional SEM image of the whole pellet from the region I. (c) Higher-magnification cross-sectional SEM image of the region II, and (d) corresponding EDS elemental Ca map. Reproduced with permission from Ref. [114], ©The Author(s) 2018. |
5.5. Development of high entropy TBCs and EBCs
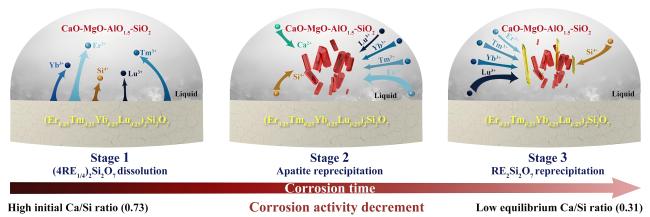
Fig. 44 Schematic diagram for the interaction between CMAS and (Er0.25Tm0.25Yb0.25Lu0.25)2Si2O7 multicomponent disilicate at 1500 ℃. Reproduced with permission from Ref. [139], © Elsevier 2022. |
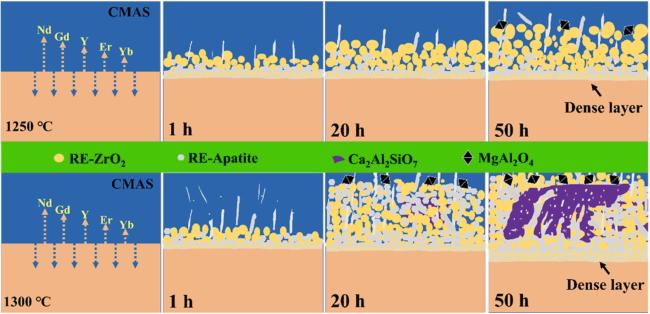
Fig. 45 Corrosion of high-entropy ceramic by CMAS at 1250 ℃ and 1300 ℃. Upper row: 1250 ℃; lower row: 1300 ℃. CMAS interaction leads to dense layers of RE-ZrO2, RE-apatite, Ca2Al2SiO7, and MgAl2O4. Reproduced with permission from Ref. [141], © Elsevier 2023. |
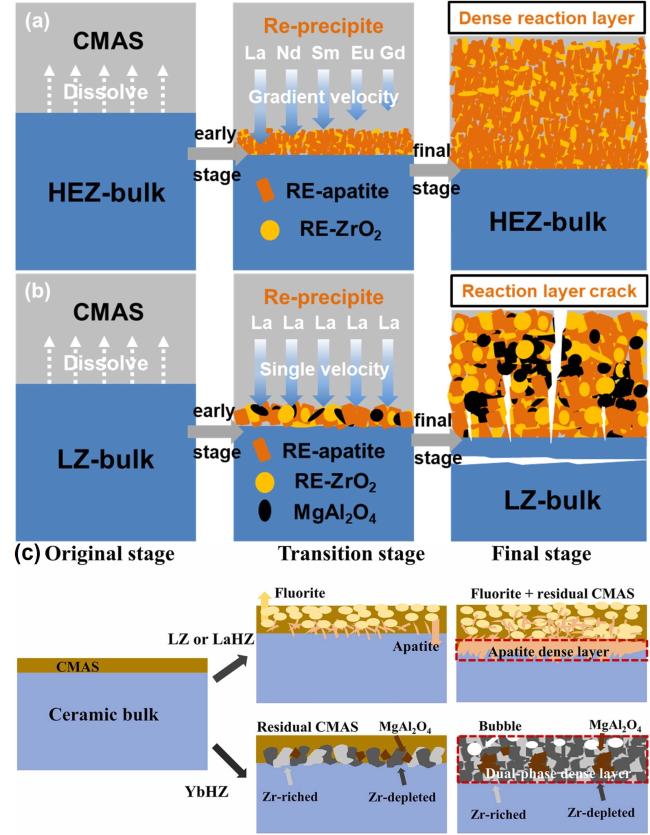
Fig. 46 CMAS-induced corrosion in HEZ and LZ ceramics. (a) HEZ: Dense RE-apatite and RE-ZrO2 layer without cracks, exhibiting graceful degradation. (b) LZ: Formation of RE-apatite, RE-ZrO2, and MgAl2O4 layer with cracks, indicating reduced CMAS resistance. Reproduced with permission from Ref. [142], © Elsevier 2022. (c) Corrosion progression of ceramic materials under CMAS exposure. Initial contact with CMAS initiates corrosion. Transition: LZ and LaHZ exhibit fluorite and apatite formation with residual CMAS and MgAl2O4. YbHZ shows the Zr-rich layer and Zr-depleted regions. Final: LZ and LaHZ develop a dense apatite layer with fluorite and CMAS residues. YbHZ forms a dual-phase layer of fluorite and pyrochlore, with enhanced CMAS resistance and no CMAS residue. Reproduced with permission from Ref. [143], © Elsevier 2024. |