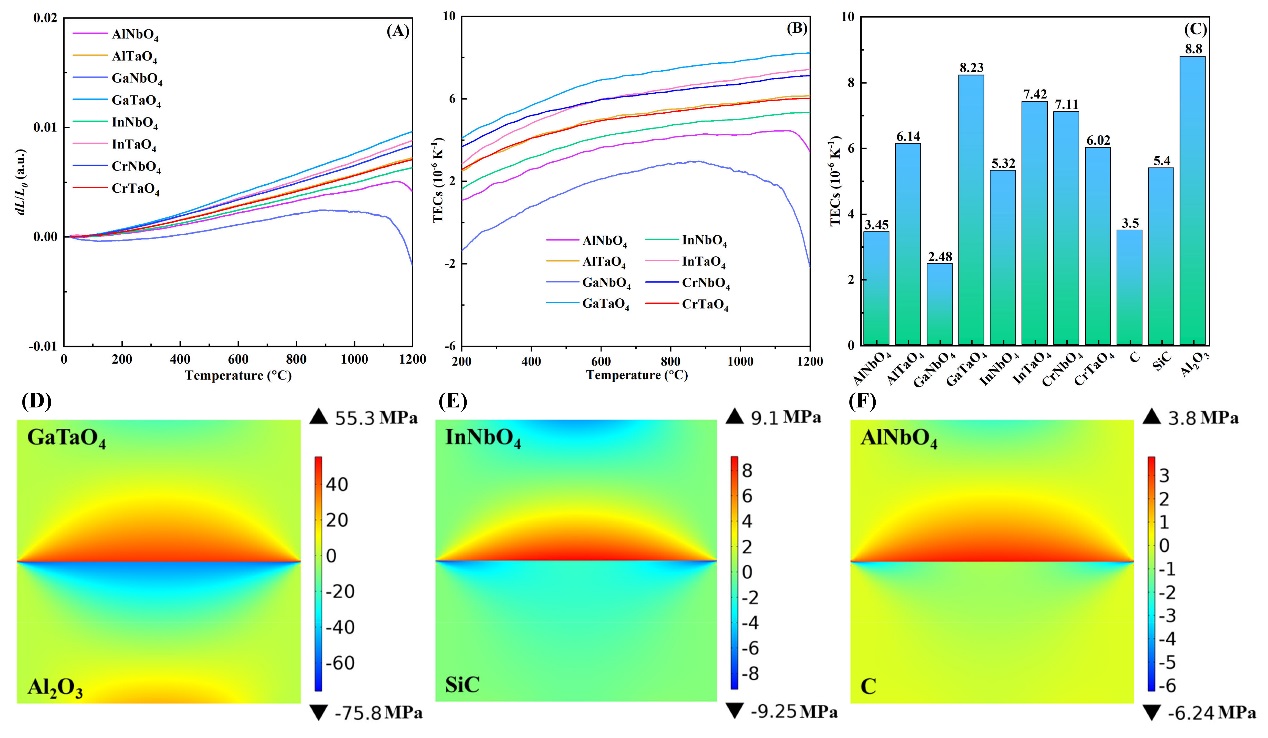
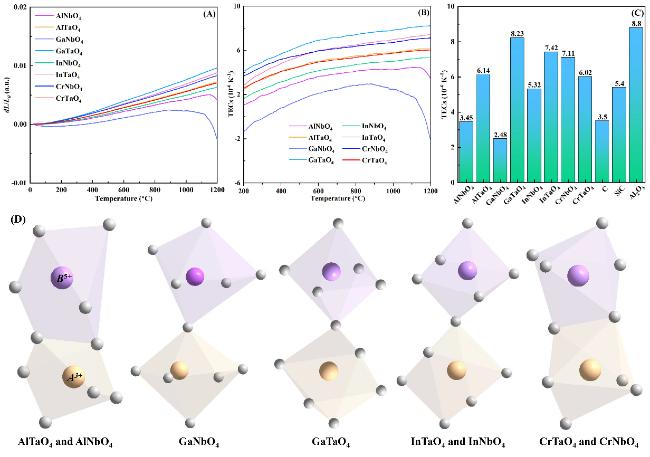
Fig. 4(A) shows the thermal expansion rate of each sample, including AlNbO
4 and AlTaO
4, which increases with the increasing temperature, except GaNbO
4 and AlNbO
4. An obvious reduction in thermal expansion rate is found in AlNbO
4 and GaNbO
4 at temperatures higher than 1150 and 1100 ºC, respectively, which is caused by their relatively low melting points. AlNbO
4 and GaNbO
4 have a melting point of 1540 ºC and 1420 ºC [
37,
38], respectively, and other
ABO
4-type oxides have higher melting points. The changes of TECs of
ABO
4-type oxides are shown in
Fig. 4(B). The TECs of GaNbO
4 decrease slightly at 900~1100 °C, while they decrease dramatically at 1100~1200 °C. GaNbO
4 has the lowest melting point among the studied oxides, and high temperatures will soften GaNbO
4 to reduce its TECs. GaTaO
4 has the highest TECs (8.23×10
-6 K
-1, 1200 ºC) among the studied oxides, which approaches those of Al
2O
3 CMCs (8.80×10
-6 K
-1), and its TECs increase with the increasing temperature. Except AlNbO
4 and GaNbO
4, the TECs of other
ABO
4-type oxides are 5.32~8.23×10
-6 K
-1 at 1200 ºC, which are suitable for EBC applications for different CMCs. C-, SiC-, and Al
2O
3-based CMCs have TECs of approximately 3.5×10
-6 K
-1, 5.4×10
-6 K
-1, and 8.8×10
-6 K
-1 at 1200 ºC, respectively as shown in
Fig. 4(C) [
39⇓-
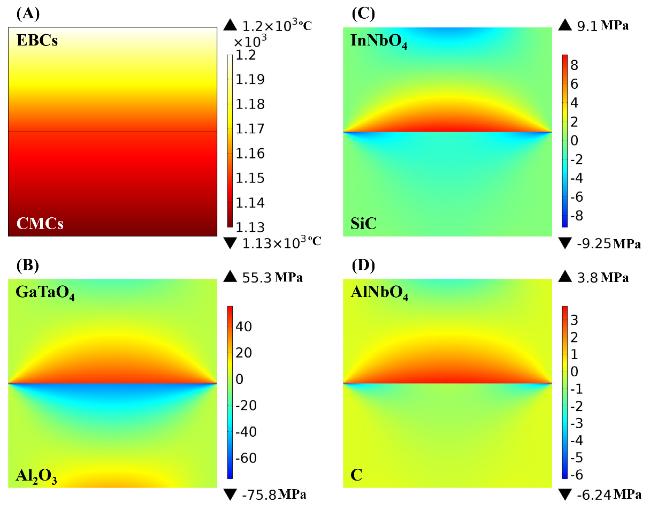
41]. The thermal stress between oxide EBCs and substrates is dominated by TECs, and it is obvious that AlNbO
4, InNbO
4, and GaTaO
4 are potential EBCs for C-, SiC-, and Al
2O
3-based CMCs, respectively.