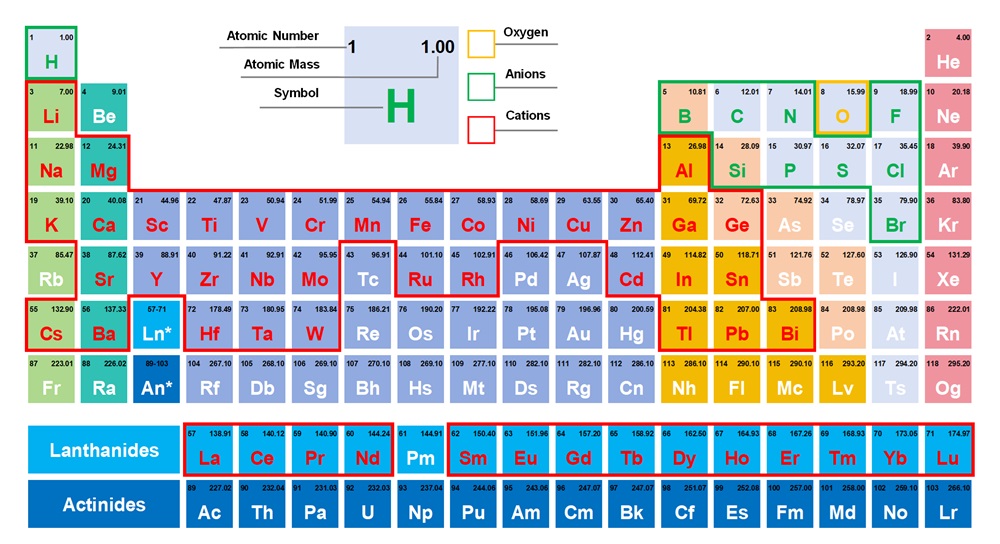
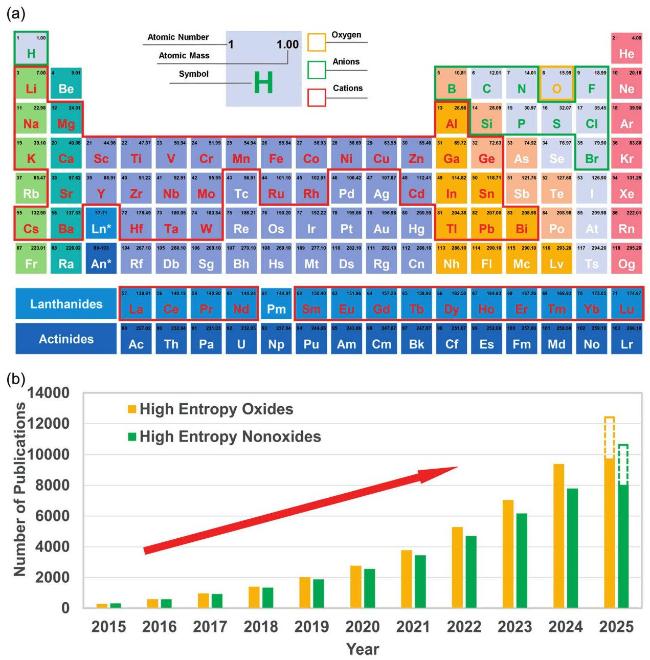
1. Introduction
Fig. 1. (a) All the constituent elements reported in HECs to date. (b) Total number of publications on major HECs over the last decade. Data was retrieved from Web of Science on February 28, 2025, using keywords: "high-entropy", "oxide", "boride", "carbide", "nitride", "silicide", "fluoride", "hydride", "phosphide", "sulfide", and "ceramics". |
2. Concept and features
Table 1 Parameters and Descriptors Related to Theoretical Design. |
| Symbol | Name | Definitional Formula | Physical Meaning | Ref |
|---|---|---|---|---|
| | Mixing Entropy | | Enthalpy change of a system when different components are mixed to form a homogeneous system. | [71] |
| | Mixing Enthalpy | | The sum of the interaction energy differences in the ideal state. | [72] |
| | Entropy-Enthalpy Balance Factor | | Tm is the isothermal temperature. | [73] |
| | Atomic (ion) Radius Difference | | Contribution of differences in atomic/ionic radii of components to lattice distortion | [74] |
| | Electronegativity Difference | | | [75] |
| VEC | Valence Electron Concentration | | Reflects the degree of electron filling and energy band structure characteristics of the system | [74] |
| DEED | Disordered Enthalpy-Entropy Descriptor | | Predicting the possibility of single-phase disordered structures in multicomponent systems, Hf and Hhull are the DFT formation energies of the partial occupation POCC tiles and the convex hull, respectively. | [76] |
| EFA | Entropy Forming Ability | | Entropy-driven formation of stable single-phase solid solutions, where n is the total number of sampled geometrical configurations and gi are their degeneracies. Hi of the sampled configurations. | [66] |
| LDI | Lattice Distortion Index | | Parameters for the degree of lattice distortion in disordered systems, ( x,y,z ) is the atomic position. | [77] |
| | The normalized geometric packing parameter for the metallic sublattice calculated | | Evaluating how different metal atoms stack together in a crystal lattice, where rS and rL are the radii of the smallest and largest atoms. | [78] |
3. Theoretical design
3.1. Stability and synthesizability
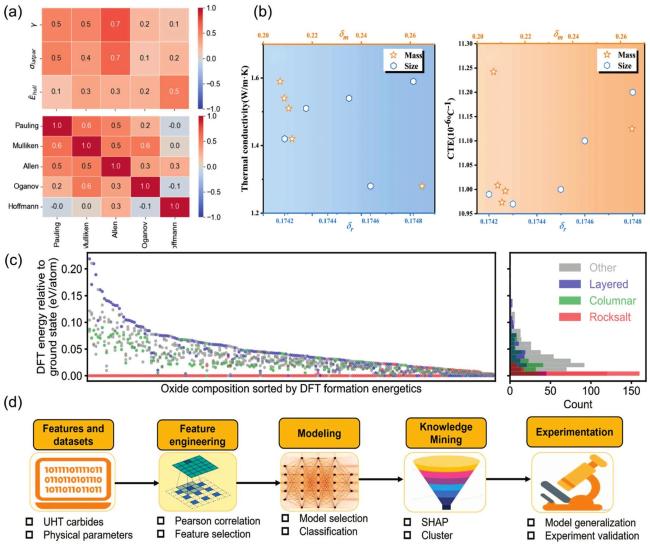
Fig. 2. (a) Pairwise correlations of computational parameters: atomic radius deviations, lattice parameters, stabilization factors for miscible binary carbides, and electrochemical factors on different scales (top); correlations between different electronegativity scales (bottom). Reproduced from Ref [64]. Copyright Elsevier 2024. (b) Atomic size difference and atomic mass difference vs. thermal conductivity (left) and thermal expansion coefficient (right) at |
et al. [67] used EFA to relate the thermal insulation properties to atomic size and mass differences to rationalize the composition of highentropy fluorite oxides (Fig. 2(b)). Further advancing this field, Peng et al. [60] established a physics-inspired descriptor based on firstness principle for predicting the degree of cation ordering in multicomponent perovskite oxides and verified that it has up to 93% accurate ordering rate in 190 experimental samples, which significantly improves the prediction of experimental ordering by machine learning and theoretical models. (Fig. 2(c)).
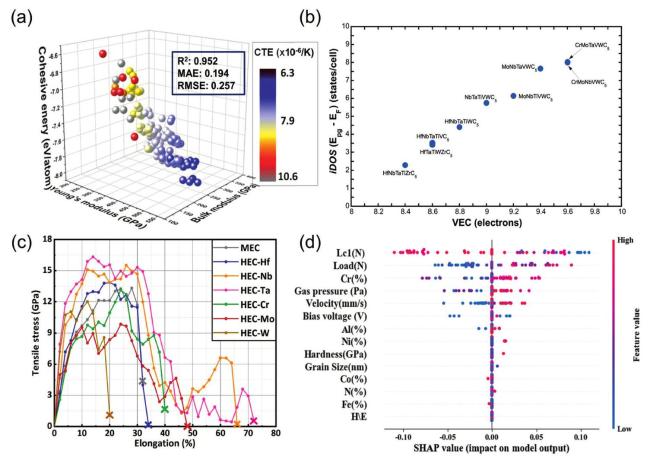
Fig. 3. (a) The 3D plots of the NET (neural network) training set were generated based on three parameters (bulk modulus, Young's modulus, and cohesive energy). Reproduced from Ref [79]. Copyright Elsevier 2024. (b) A plot of (iDOS( Epg,EF )) vs. VEC composed of nine solid solution high-entropy carbides shows that iDOS increases linearly along with VEC, and Pearson's correlation coefficient was 0.99. Reproduced from Ref [82]. Copyright Elsevier 2024. (c) The stress-strain curves from the AIMD (Ab Initio Molecular Dynamics) tensile simulations of MEC and HEC. Reproduced from Ref [77]. Copyright Elsevier 2024. (d) SHAP (Shapley Additive exPlanations) visualization features importance ranking. Reproduced from Ref [84]. Copyright Elsevier 2024. |
3.2. Properties prediction
4. Structures and compositions
4.1. High-entropy simple oxides
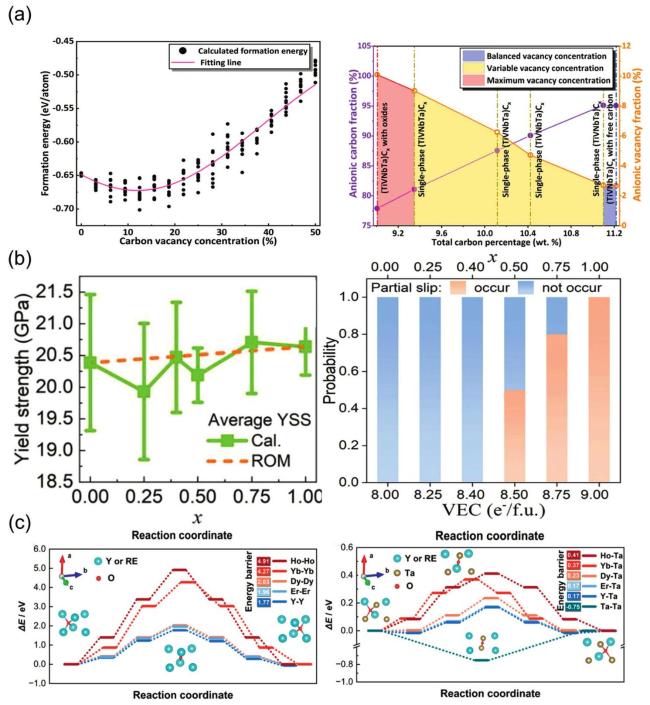
Fig. 4. (a) The fitting formation energy curve of (TiVNbTa)Cx with variation of anionic vacancy concentration; Curves of anionic carbon and vacancy fraction under changing carbon content. Reproduced from Ref [86]. Copyright Elsevier 2024. (b) Average YSSs and ROM (rule of mixture) predictions for (HfTiZr) |
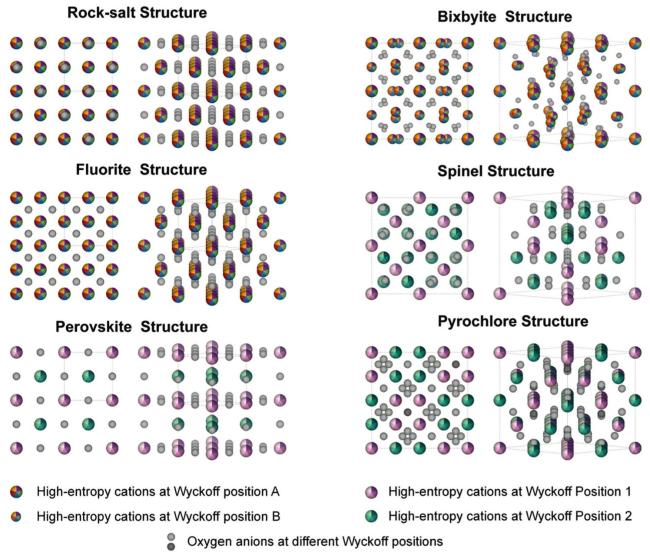
Fig. 5. Typical crystal structures of high-entropy ceramics. Here, two color schemes are used to clarify the difference between simple and complex oxides, and 2×2×2 supercells for the three crystal structures at left side for better comparison. |
Table 2 Synthesis methods for high-entropy simple oxides corresponding product features. |
| Composition | Preparation | Feature | Ref. |
|---|---|---|---|
| (MgCoNiCuZn)O | Pulsed Laser Deposition (PLD) | structural stability of rock-salt modulated by Mg, Ni, Co | [94] |
| Li(CrMnFeCoNiCu)O2 | single-phase layered rock-salt oxide films | [95] | |
| | Solid-state reaction | [96] | |
| | single-phase cubic fluorite structure uniformly distributed fluorite structure with good force thermal stability | [97] | |
| (ZrHfTiSn)O2 | Solid phase sintering | highly resistant to irradiation | [98] |
| RE2 A2O7(RE=[La,Sm,Nd,Gd,Dy,Yb,Y,Er,Eu, Tb, Ho, Lu, Tm], | RE2Ce2O7 and RE2Pr2O7 exhibit a single fluorite structure | [99] | |
| | uniform fluorite structure | [100] | |
| (CuCoNiZnMg)O | Coordination Polymerization Solid Dispersant-Assisted Annealing | single-crystalline HEO rods with uniform dispersion | [101] |
| (BiZrMoWCeLa)O2 | Surfactantassisted hydrothermal technique | high electrochemical efficiency | [102] |
| (YLaNdSmYbLuEuGdCe))2O3 | Co-precipitation method combined with conventional sintering | bixbyite structure with high oxygen barrier properties | [103] |
| | Solution combustion synthesis method combined with conventional sintering Spark Plasma Sintering (SPS) | with cubic bixbyite-structured, and better oxygen barrier properties. | [104] |
| | Mechanochemical ball milling and sol-gel | the highest properties in the sol-gel process, followed by that in wet milling and dry milling | [106] |
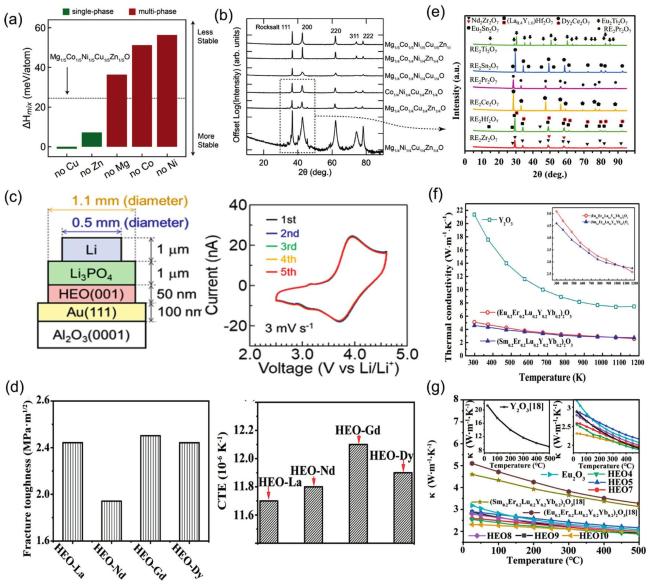
Fig. 6. (a) X-ray diffractograms of four-component derivatives of (MgCoNiCuZn)O as a substrate; (b) DFT analysis of four diffractive phase compositions by mixing enthalpy. Reproduced from Ref [94]. Copyright Elsevier 2024. (c) Side diagram of thin film battery with 50 nmLi(CrMnFeCoNiCu)2 (001) as positive electrode, and cyclic voltammogram. Reproduced from Ref [95]. Copyright American Chemical Society 2022. (d) CTE and Fracture toughness of HEO-La, Nd, Gd, Dy. Reproduced from Ref [97]. Copyright Elsevier 2024. (e) the XRD patterns of the samples. It can be seen that RE2Zr2O7 and RE2Hf2O7 failed to form single-phase high-entropy pyrochlore phase; RE2Ce2O7 and RE2Pr2O7HEOs, exhibited a single phase structure with a fluorite-type structure. Reproduced from Ref [99]. Copyright Elsevier 2025. (f) Calculation of thermal conductivity of (EuErLuYYb)2O3,(SmErLuYYb)2O3 and Y2O3. Reproduced from Ref [105]. Copyright Tsinghua University Press 2021. (g) Thermal conductivity |
4.2. High-entropy complex oxides
Table 3 Synthesis methods for high-entropy complex oxides corresponding product features. |
| Composition | Preparation | Feature | Ref. |
|---|---|---|---|
| Pb(TiZrHfNbAl)-O | Solid-state reaction | show powdery HEOs and the perovskite phase | [115] |
| Pb(TiZrHfNbCr)-O | formation is consistent with the Goldschmidt | ||
| Pb(TiZrHfNbMn)-O | |||
| Pb(TiZrHfNbSc)-O | |||
| (BiBaSrCaNa)Ti-O | |||
| (BiBaSrCaK)Ti-O | |||
| (BiBaSrNaPb)Ti-O | |||
| (AlCoFeNiTi)3O4 | with anti-spinel structure | [116] | |
| Ba(ZrTiSnHfX)O3(X=Nb5+,Ta5+) | exhibits exceptional thermal stability within the range of 30 to | [117] | |
| Ca5Sr5Ba5 Pb5Nb2O6 | unfilled tetragonal tungsten bronze (TTB) structured | [118] | |
| | the Ce-component samples showed superior | [119] | |
| Ba0.4 Sr0.6-xCaxNb2-xTaxO6 | exhibits TTB structure with significant dielectric properties | [120] | |
| (YbLuTm)2Si2O7-(YbLuTmErHoDyGdSm)2Si2O7(3-8HESi2O7) | High-throughput pressure-less sintering | excellent corrosion resistance at 1773 K | [121] |
| (MnCoNiCuX)Fe2O4(X=Fe,Mg) | Reactive flash sintering | a spinel crystal structure was obtained in just 30 min at 1173 K | [122] |
| Sr(Ti2Zr2Hf3Mn0.15Sn0.18)O2.85 | Multimetallic polymeric precursors and photolithographic additive manufacturing | retain the printed geometry with high shape fidelity | [123] |
| La(FeCuMnMgTi)O3 | Solid-state milling-heating method | displays robust stability in benzyl alcohol oxidation. | [124] |
| (YbTmLuHoEr)2Ti2O7 | Floating-zone growth technique | monocrystalline pyrochlore structure | [125] |
| (YGdDyErYb)2Hf2O7 | solution combustion | single phase fluorite structure with good TBC performance | [126] |
| | Sol-gel method | outstanding high temperature stability and CMAS corrosion resistance | [127] |
| (YDyErTmYb)4Hf3O12 | ultrafast high-temperature sintering (UHS) | extremely sluggish grain growth characteristics and excellent high-temperature phase stability | [128] |
| (YYbLuEuEr)3Al5O12 | Laser powder bed fusion | high-entropy phase with dendritic morphology | [129] |
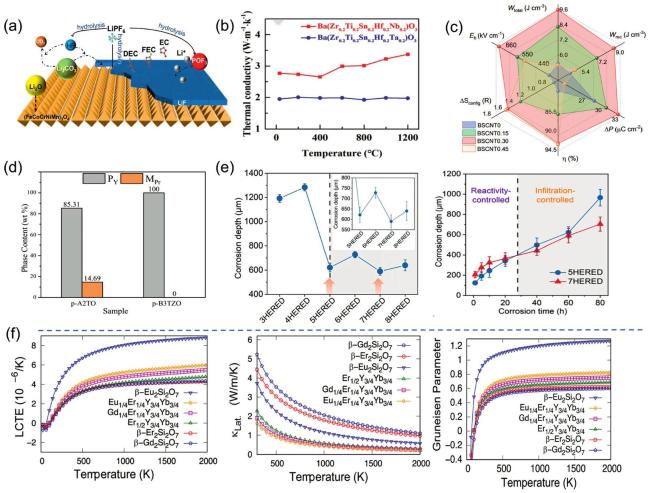
Fig. 7. (a) Schematic diagram representation of the conversion of Li2O,LiF, and Li2CO3 during the cycling process of the preconditioned HEOs. Reproduced from Ref [112]. Copyright Elsevier 2024. (b) Comparison of thermal properties at X=Ta,Nb. Reproduced from Ref [117]. Copyright The Royal Society of Chemistry 2024. (c) Effect of different configurational entropy (different x ) on energy storage performance. Reproduced from Ref [120]. (d) Effect of the introduction of Zr4+ on the content of the pyrochlore phase (PY) and monoclinic phase (MPr). Reproduced from Ref [137]. Copyright Elsevier 2023. (e) Corrosion depths of different component HEREDs at 1673 K,60 h; time-dependent curves of corrosion depths of 5HERED vs. 7HERED at 1673 K. Reproduced from Ref [121]. Copyright Elsevier 2024. (f) Thermal conductivity, CTE, and Grüneisen parameters as a function of temperature. Reproduced from Ref [140]. Copyright The Royal Society of Chemistry 2024. |
Table 4 Synthesis methods for high-entropy non-oxides corresponding product features. |
| Composition | Preparation | Feature | Ref. |
|---|---|---|---|
| (HfZrMoMoNbTi)B2 | High-energy ball milling and spark plasma | possess one solid-solution boride phase of the | [143] |
| (Hf, Zr, Ta, Sm)B 2 | Boron thermal reduction and hot press sintering | favorable oxidation resistance | [144] |
| (Hf0.28Zr0.28Ta0.28RE0.16)B2 | Ultrafast-UHS and SPS | HEB2-Sc has the best resistance to oxidation | [145] |
| | One-step in-situ carbo-thermal reduction and | x=0.75-0.85 range formed a stable two-phase | [146] |
| | pressureless vacuum sintering | HEC-Ni ceramic metal | |
| (TiZrNbTaCr)C | Carbothermal reduction reaction | Cr addition is beneficial to the oxidation resistance | [147] |
| (Zr,Nb,Ta,Ti,W)C | Selective laser sintering (SLS) | showed enhanced hardness and reduced thermal conductivity, | [148] |
| (CrNbTaMoW)C0.83 | Ultrafast Pressure Sintering (UPS) | dense single-phase and homogeneous structure in 3 min | [149] |
| (Zr-Nb-Hf-Ta) C1-x Nx | SPS | higher OOT compared to high entropy carbides and nitrides | [150] |
| MAX-phase ( | higher room and high temperature plasticity | [151] | |
| (MoWCrTaNb) Si2 | micron-scale uniform C40 hexagonal structure | [152] | |
| ( MoNbTaTiZr )1-x Nx | Hybrid direct current magnetron sputtering | x=0 presents a BCC structure, x=0.17 presents a FCC structure | [153] |
| ( Ti,Zr,Nb,Mo,Ta)C1-x Nx | Open dynamic carbothermal reduction nitriding | HEC0.9 N0.1 exhibits the highest mechanical properties | [154] |
| K0.65Li0.07Mg0.19Mn0.17Co0.16Ni0.17Cu4 F2.70 | Direct liquid-phase method | higher battery capacity | [155] |
| (LaCePrNdSmEuGdDyHoErYbScY)OCl | In-situ core@shell@shell interdiffusion strategy | significant bandgap modulation effects | [156] |
4.3. High-entropy non-oxides
4.3.1. High-entropy Carbides
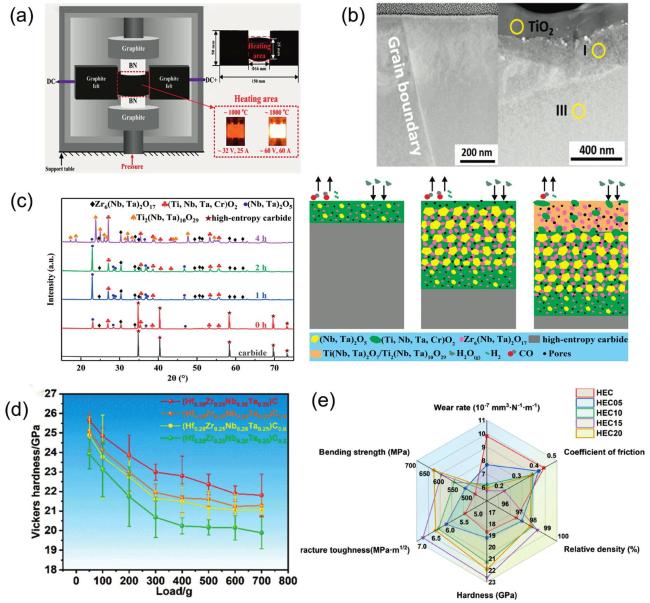
Fig. 8. (a) Schematic diagram of ultra-fast pressure sintering apparatus. Reproduced from Ref [149]. Copyright Elsevier 2025. (b) TEM cross-sections of the two samples sintered by SPS and SLS respectively, the sample with SPS has a uniform morphology and the sample sintered by SLS has a three-layer structure. Reproduced from Ref [148]. Copyright American Ceramic Society 2024. (c) XRD patterns of the samples oxidized for different durations, and Ti2(Nb,Ta)10O29 appeared at 4 h ; The schematic diagram of the oxidation mechanism of (TiZrNbTaCr)C in steam at |
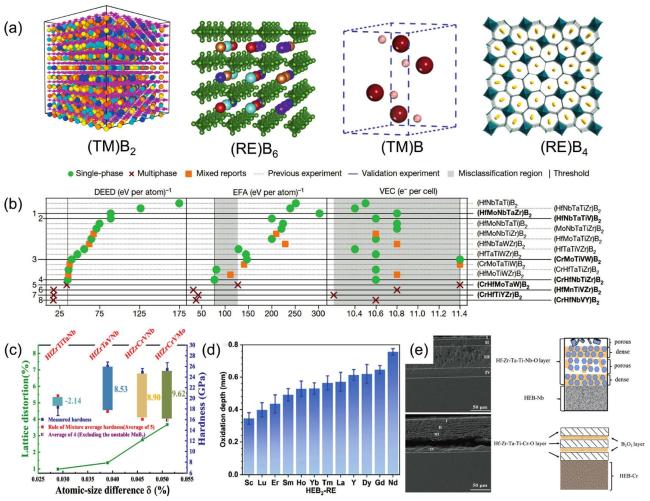
Fig. 9. (a) Crystal structures of different HEBs. Reproduced from Ref [167,170-172]. Copyright Elsevier 2021. (b) Prediction of multiple HEB2 syntheses by DEED: Compared with the results of EFA and VEC, the prediction of DEED was significantly better than the other two. Reproduced from Ref [10]. Copyright The Nature Publishing Group 2024. (c) Correlation between hardness, lattice distortion, and Atomic-size difference |
4.3.2. High-entropy borides
4.3.3. High-entropy nitrides
4.3.4. Other high-entropy ceramics
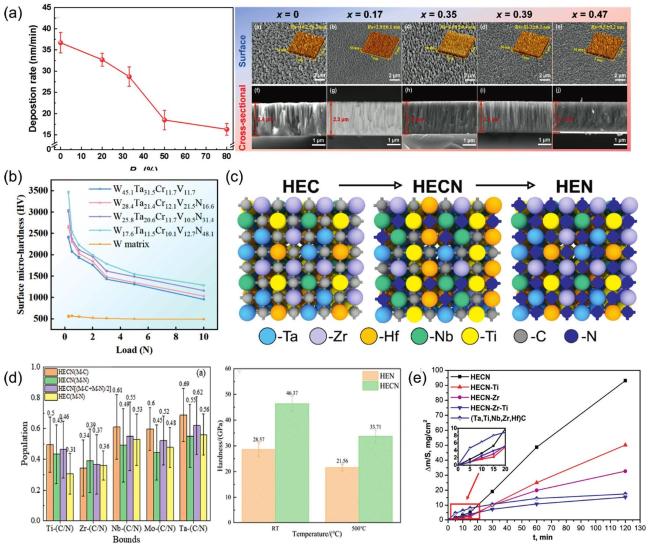
Fig. 10. (a) SEM images of surface and cross-sectional morphology of (MoNbTaTiZr) 1-x Nx coatings under different x; Deposition rates of (MoNbTaTiZr) 1-x Nx coatings with different RN. Reproduced from Ref [177]. Copyright Elsevier 2025. (b) Surface micro-hardness of W-Ta-Cr-V-N coatings. Reproduced from Ref [176]. Copyright Elsevier 2024. (c) Crystal Structures of HEC, HECN, and HEN. Reproduced from Ref [174]. Copyright Elsevier 2024. (d) Bond populations and density of states for HEN and HECN coatings; Crystal Structures of HEC, HECN, and HEN. Reproduced from Ref [179]. Copyright Elsevier 2024. (e) The increase in mass per unit area as a function of oxidation time reflects the variation of the surface mass of the sample with time at different oxidation durations. |
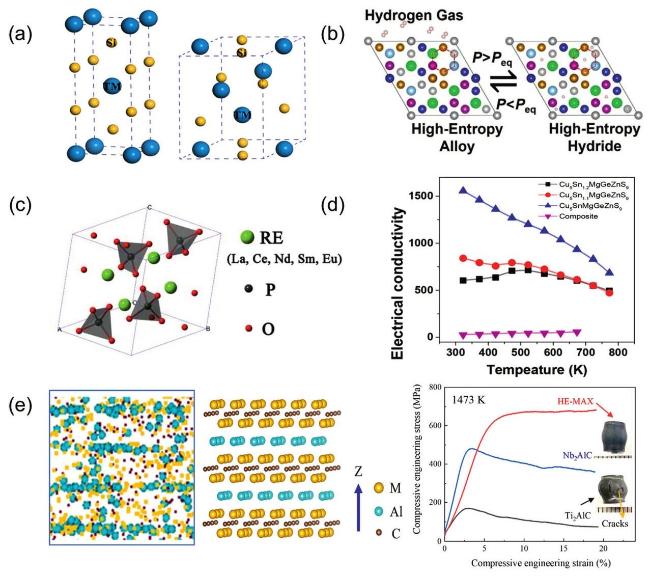
Fig. 11. (a) Crystal structure of tetragonal transition metal disilicide (left), hexagonal transition metal disilicides (right). Reproduced from Ref [196]. Copyright Elsevier 2023. (b) Reversible phase transitions of HEA and HEH during hydrogen absorption. Reproduced from Ref [190]. Copyright Elsevier 2024. (c) Crystal structure of HEPO4 monazites. Reproduced from Ref [191]. Copyright Elsevier 2019. (d) Electrical conductivity of the high-entropy sulfides. Reproduced from Ref [192]. Copyright American Chemical Society 2018. (e) A tomogram slices of the HE-MAX phase lattice structure as well as a picture of the crystal structure in the ideal case; Compressive stressstrain curves of the HE-MAX, Nb2AlC, and Ti2AlC at 1473 K, insets show the specimens after testing is completed. Reproduced from Ref [151]. Copyright Elsevier 2024. |
5. Properties and applications
5.1. Mechanical properties
Table 5 Summary of properties of high-entropy ceramics. |
| Categories | Materials | Key Findings | Ref |
|---|---|---|---|
| Mechanical | (Al1/6Cr1/6Nb1/6TaTi1/3)O2 | elastic modulus ( | [197] |
| HE TM 0.8Sc0.2 B2 | B=254GPa | [198] | |
| HE TM 0.75Sc0.25 B2 | G=237GPa | ||
| HE TM 0.75Lu0.25 B2 | E=539GPa | ||
| (HfMoNbTaTi)C | low friction and wear ( 0.1 and 107 mm3/Nm ) | [199] | |
| H=18.7GPa | |||
| (WTaNbZrTi)C | H=21.0GPa | [14] | |
| ( CeZrLaSmNdY | KIC=8.07MPa⋅m1/2 | [96] | |
| (TiTaNbZr)C | | [13] | |
| Thermal | | | [200] |
| | | [201] | |
| lattice thermal conductivity of 2.54 W/(m⋅K) at 1073 K | |||
| ⟨001⟩-textured (LaSrBaCa) 0.85TiO3 | ZT=0.13 | [203] | |
| Zr0.279(Y0.0708Yb0.0302Ta0.0329Nb0.0402)O0.5469 | CTE=10.6∼10.9×10-6 K-1 (at | [204] | |
| (LaNdSmEuGd)CrO3 | | [205] | |
| Electircal | | maximum power density of 2.03 W/cm2 | [206] |
| | PPD=1.33 W/cm2 | [207] | |
| LiNi0.8Co0.15Al0.05O2 | Coulombic efficiencies of Li-ion batteries over 99.9 % | [208] | |
| (MgTiZnCuF)3O4 | Reversible capacity of 504 mA h/g | [209] | |
| | Electrical conductivity 635.15 S/cm at | [210] | |
| | Initial discharge capacity of 125.9 mA h/g | [211] | |
| Catalytic | (FeMnCoNiCr)3O4 | PPD=1.33 W/cm2 at solid oxide fuel cells | [33] |
| (TiVCrMo)B2 | FE=97.9% (at hydrogenation process of NO3 -RR ) | [212] | |
| | overpotential of 40.7 mV at 10 mA/cm2 | [21] | |
| (FeCoNiCuZn)3O4 | HER ( | [20] | |
| Ru0.13/Ba0.3Sr0.3Bi0.4(ZrHfTiFe)O3 | 51%CO conversion at | [49] | |
| Magnetic | (CrMnFeCoNi)3O4 | ferrimagnetic behavior with a saturation magnetization of 22emu/g at 2 K and 7.2emu/g at 300 K | [35] |
| LaCr0.2Mn0.2Fe0.2Al0.2Ga0.2O3 | Magnetic behavior driven by competing interactions among Cr,Mn, and Fe sublattices | [34] | |
| (LaNdSmGd)1-xYbxMnO3 | | [56] | |
| Dielectric | SrLa(Al0.50-xGaxZn0.125Mg0.125Ti0.25)O4 | Qf=98,000GHz | [214] |
| (MgCoNiCuZn)O, | PD=332.88MW/cm3 | [215] | |
| 0.8Na0.5Bi0.5TiO3-0.2Sr(ZrSnHfTiNb)O3 | high | [217] | |
| | Wrec =4.89 J/cm3,g=91.2% | [218] |
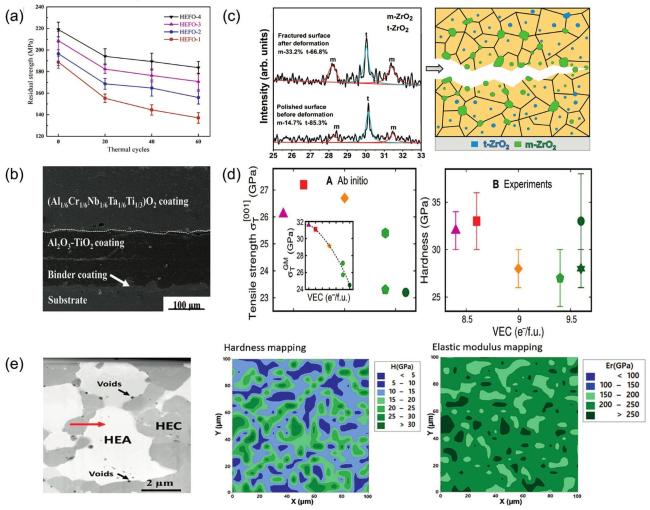
Fig. 12. (a) Residual flexural strength after cyclic thermal shock at |
5.2. Thermal properties
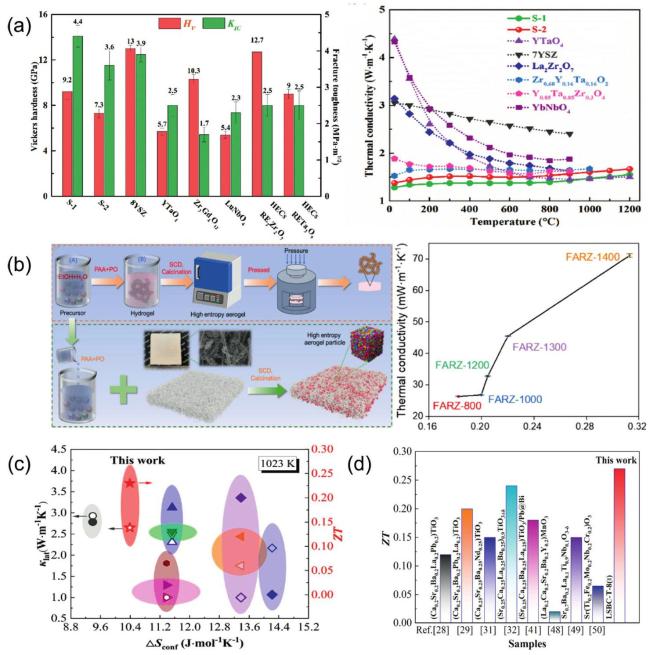
Fig. 13. (a) Mechanical property comparisons between dual-phase zirconate/tantalate HECs, 8YSZ, YTaO4, and others. Reproduced from Ref [204]. Copyright Elsevier 2024. (b) The fabrication process of (YErYbGdLa) 2Zr2O7 ceramic aerogel and its thermal insulating properties. Reproduced from Ref [52]. Copyright Elsevier 2024. (c) Comparison of PF (power factor) and ZT of ceramics with reported high-entropy perovskites at x=0.10. Reproduced from Ref [202]. Copyright Elsevier 2023. (d) Comparison of the ZT in this study with those of previously reported high-entropy perovskites. Reproduced from Ref [203]. Copyright Elsevier 2024. |
5.3. Electrical properties
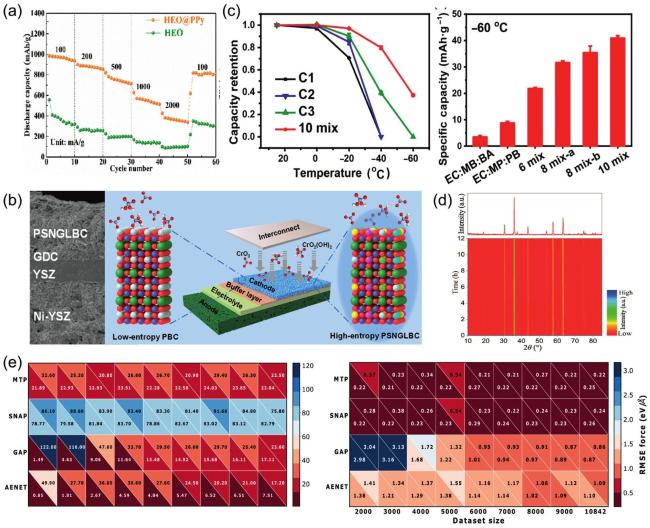
Fig. 14. (a) The rate discharge performance of HEO and HEO@PPy at various current densities; the rate performance of HEO@PPy significantly outperforms that of HEO. Reproduced from Ref [224]. Copyright Elsevier 2024. (b) Schematic and SEM cross-section of a single cell with (PrSmNdGdLa)BaCo |
Fig. 15. (a) Temperature-dependent ( |
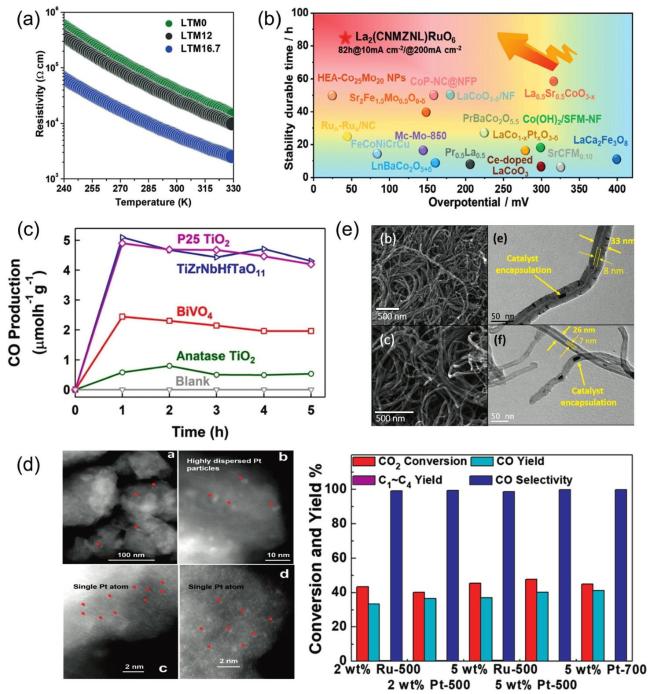
5.4. Catalytic properties
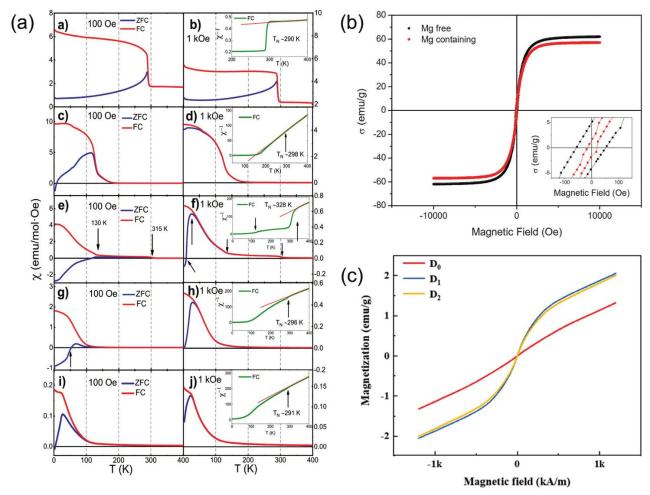
Fig. 16. (a) Magnetic induction strength versus temperature for (a-b) LC, (c-d) LCM, (e-f) LCMF, (g-h) LCMFA, and (i-j) LCMFAG ceramics in ZFC (Zero Field Cooling) and FC (Field Cooling) modes at 100 Oe and 1000 Oe, inset show the |
5.5. Magnetic properties
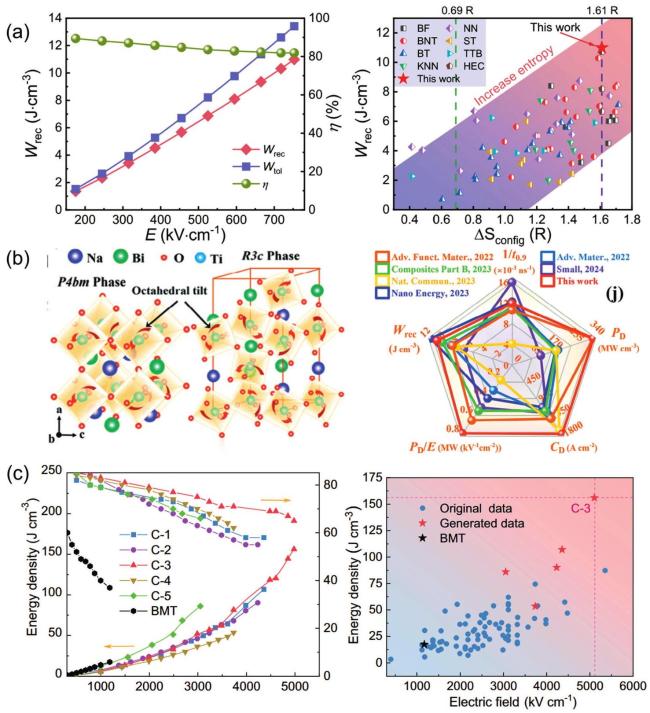
Fig. 17. (a) Wrec and |
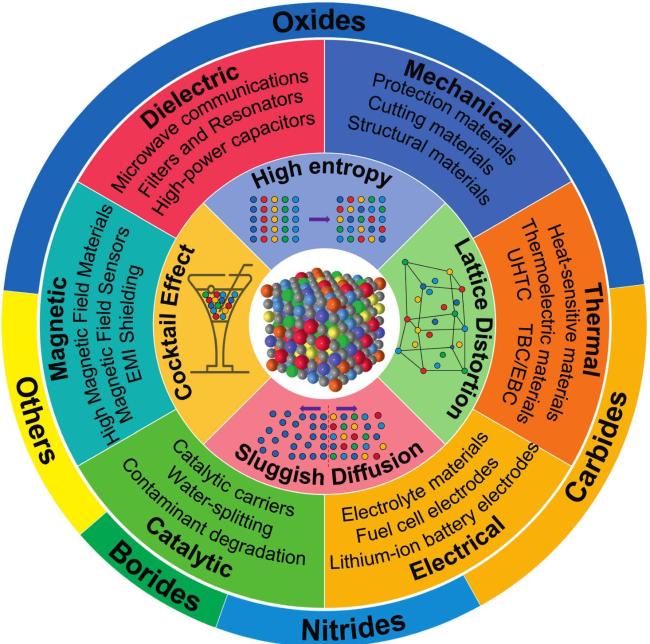
Fig. 18. An overview diagram of HECs. The outer ring shows the percentage of studies on high-entropy oxides, carbides, nitrides, borides and others. The inner rings show the main applications of HECs, as well as the four core effects of high-entropy materials and a schematic of the disordered structure, respectively. |